CDC warns deadly bacterial infection may be spreading in eye drops made in India
At least one person has died from drug-resistant bacterial infection
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
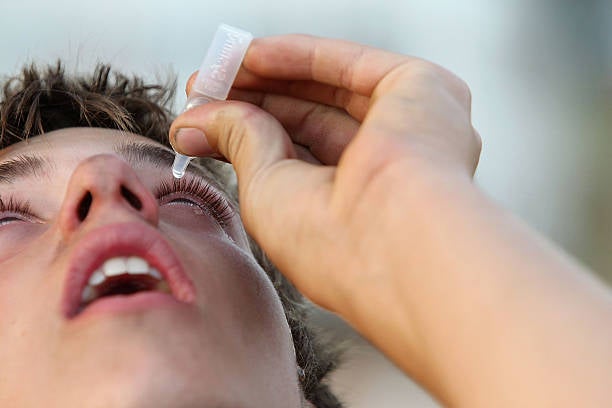
Your support makes all the difference.The US Centers for Disease Control and Prevention has advised against the use of an over-the-counter eye drop manufactured in India after finding that it may be linked to a bacterial infection leading to hospitalisation, permanent vision loss, and death.
The CDC has identified at least 55 people in 12 states with Pseudomonas aeruginosa, a type of bacteria resistant to most antibiotics. So far, one person has died and three people had permanent vision loss.
While the agency has not traced the infection to the eye drop Artificial Tears, it said that a majority of people who became ill reported using it.
“CDC recommends that clinicians and patients immediately discontinue the use of EzriCare Artificial Tears until the epidemiological investigation and laboratory analyses are complete,” it said.
Among the reported cases, at least 11 developed eye infections, while three experienced permanent vision loss in one eye. There were also those who had respiratory infection or urinary tract infections while one person lost their life after the bacterium entered the bloodstream, reported Fox News.
The cases have so far been found in California, Colorado, Connecticut, Florida, New Jersey, New Mexico, New York, Nevada, Texas, Utah, and Washington.
The eye drops are preservative-free, meaning they do not have ingredients to prevent the growth of bacteria. According to the agency, the infection could have arised from contamination, either during the use or while their manufacture.
CDC is testing unopened bottles, reported CNN.
New Jersey-based EzriCare issued a statement saying it has taken “action to stop any further distribution or sale” of the eye drop.
“As of today, we are not aware of any testing that definitively links the Pseudomonas aeruginosa outbreak to EzriCare Artificial Tears,” it said, adding that they are cooperating with the health agencies and have also contacted customers to advise them against the use of the product.
The company disclosed that the EzriCare Artificial Tears product is manufactured in India by Global Pharma Healthcare PVT Limited and imported into the United States by Aru Pharma Inc.
“EzriCare, LLC had no role in the formulation, packaging delivery system design or actual manufacturing of this product”.
“We understand that Global Pharma Healthcare PVT Limited will be initiating a recall of the product, but as of the date and time of this Press Release that has not happened,” the company said, adding that it has not happened so far.
The Chennai- based manufacturers confirmed to The Independent that they have taken steps to recall the products but it has been done only for those sent to the US.
“We are in touch with the FDA and have begun the recall procedure,” said Global Pharma’s general manager Selvam Kalyanadundaram.
Elaborating on the internal measures taken since the matter was brought to the pharmaceutical’s notice, the company said they have also begun a probe.
“We have started our investigation. We have sent the material to the lab. But as per the interaction with the FDA team, we said we will voluntarily recall the product because we are not going to wait for the results.”
“We have started investigation to look for what is the root cause because [the FDA] said, it is a microbial contamination. The microbial contamination may acquire at different places. We don’t want to make any argument and that is why we said, we will do a voluntary recall (sic),” he said.
This is the latest international scandal involving an Indian pharmaceutical. Earlier this year, India suspended the production license of Marion Biotech, whose cough syrup has been linked to the death of 19 children in Uzbekistan.
Four made-in-India syrups were also linked to the deaths of 66 children in Gambia last year. In a letter to the World Health Organisation (WHO), India said that its cough syrups were “prematurely” linked to the deaths of children which “adversely impacted” the image of the country’s pharma industry.
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments