India says WHO prematurely linked its cough syrups to deaths of children in Gambia
India says some of the children who died had not consumed the syrup
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.India has told the World Health Organisation (WHO) that its cough syrups were “prematurely” linked to the deaths of children in Gambia which “adversely impacted” the image of the country’s pharma industry.
In a letter to WHO’s regulation and prequalification director Dr Rogerio Gaspar, the drugs controller general of India (DCGI) Dr V G Somani said that Gambian authorities found no direct causal relationship between the India-made cough syrup and the deaths of children.
It also said that Gambia has informed that some of the children who died had not consumed the syrup, according to Indian media reports.
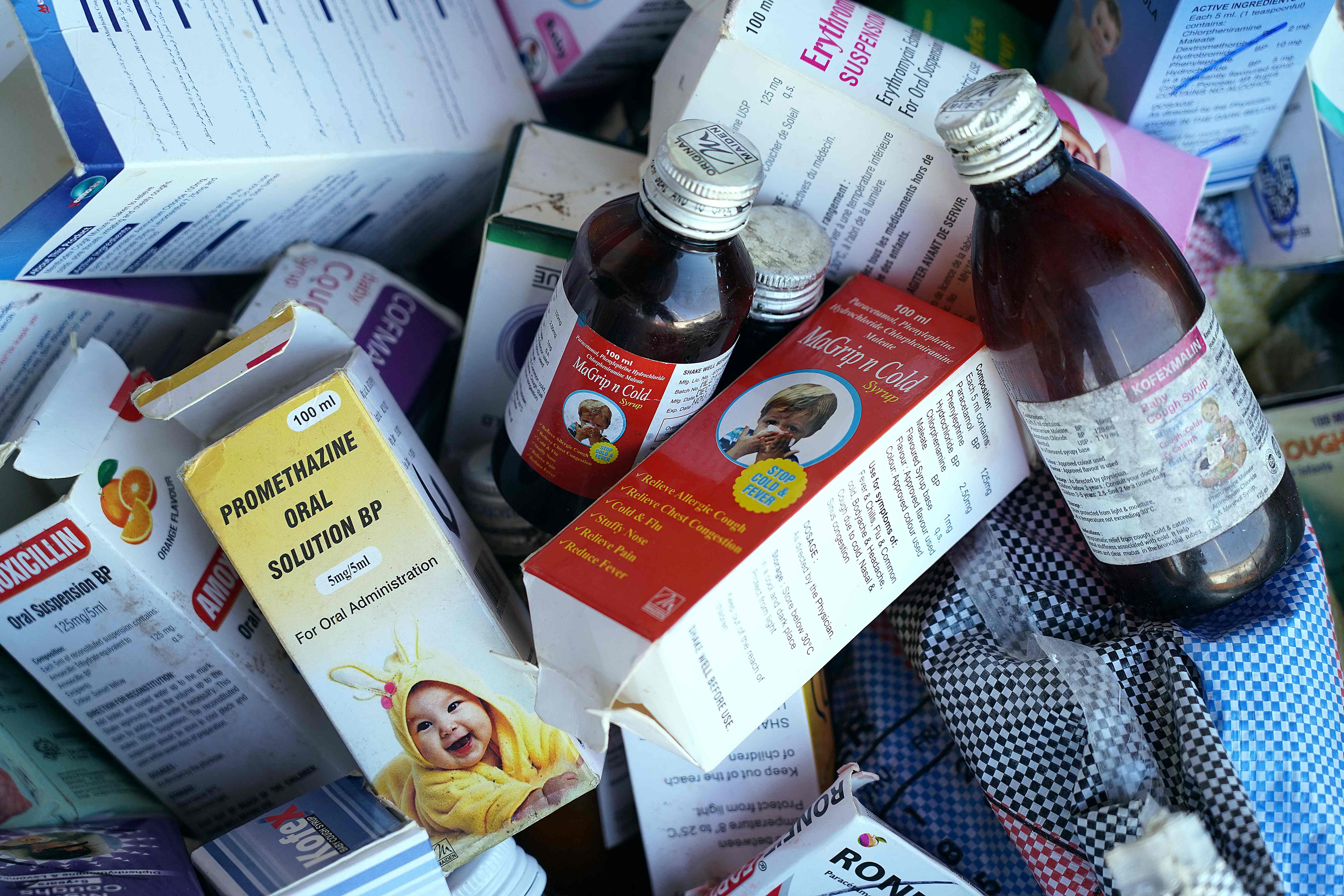
Four made-in-India syrups were linked to the deaths of 66 children in Gambia earlier this year, with WHO reaching out to Indian authorities for checking the quality and safety of the products, sparking an investigation against Maiden Pharmaceuticals which had to halt its production hub in northern India.
Mr Somani, however, wrote that the testing of the samples of four syrups in question, carried out in government-owned labs, found no contamination.
“It is clear that perhaps premature deduction was drawn on September 29th itself regarding the cause of death. Every subsequent alert or publication from the WHO only seems to be a reaffirmation of this deduction, without waiting for independent verification,” Mr Somani said.
The DGCI in the letter said that the allegations of contamination levelled by WHO were “unfortunately amplified by the global media which led to a narrative being built internationally targeting the quality of Indian pharmaceutical products”.
“This in turn has adversely impacted the image of India’s pharmaceutical products across the globe, and caused irreparable damage to the supply chain of pharmaceutical products, as well as repute of the national regulatory framework over an assumption that has yet not been substantiated by the WHO or its partners on ground,” the DCGI said.
The reports have now been sent to a technical committee of experts, Indian news channel NDTV reported.
The DCGI wrote that India will continue to provide full cooperation and collaboration with WHO and the results will be shared with the international health body.



Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments