Landmark sickle cell drug axed by regulators
Exclusive: Crizanlizumab was hailed as first new drug to be introduced in over 20 years for people living with sickle cell disease
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.A drug approved to treat sickle cell disease has been axed by the government, sparking criticism from campaigners.
The medicine, crizanlizumab, which was rolled out in 2022, was hailed as the first new drug to be introduced in more than 20 years for people living with the blood disease, which mainly affects Black, Asian and ethnic minority heritage groups.
But after an internal review, the Medicines and Healthcare Products Regulatory Agency (MHRA), an agency within the Department of Health and Social Care, confirmed it had been scrapped.
The decision was slammed by campaigners.
John James, from the Sickle Cell Society, said: “Given that sickle cell disorder is currently the fastest growing genetic disorder in the UK, with nearly 300 babies born with the condition each year, it is appalling that we have gone backwards to a reliance on only one licensed NHS treatment.”
Though no safety concerns were identified, the MHRA concluded the drug is not sufficiently effective, despite evidence suggesting that it eases symptoms for those living with the illness.
Some 5,000 eligible sickle cell patients will have to do without this treatment and the MHRA told The Independent that at least 200 patients were already being treated with this drug.
Mr James went on: “We understand the complexities involved in regulatory decisions, but we also urge for continued research and exploration of alternative solutions to address the unmet needs of patients.
“The MHRA decision is a setback, but we remain hopeful for the future.”
This move means that with immediate effect, no new patients will be prescribed crizanlizumab, and those already on the drug will need to discuss treatment options with their doctors.
Sickle cell disease can damage organs and cause intense pain, as well as anaemia because the blood cells cannot carry oxygen effectively around the body, leaving sufferers with tiredness and shortness of breath.
It is a serious and lifelong health condition, often requiring hospital admissions. Living with this illness can make it difficult for many patients to continue in their jobs or other everyday activities.
Guidance from the National Institute for Health and Care Excellence (NICE) recommended crizanlizumab as an effective treatment for reducing chronic pain while improving patients’ quality of life.
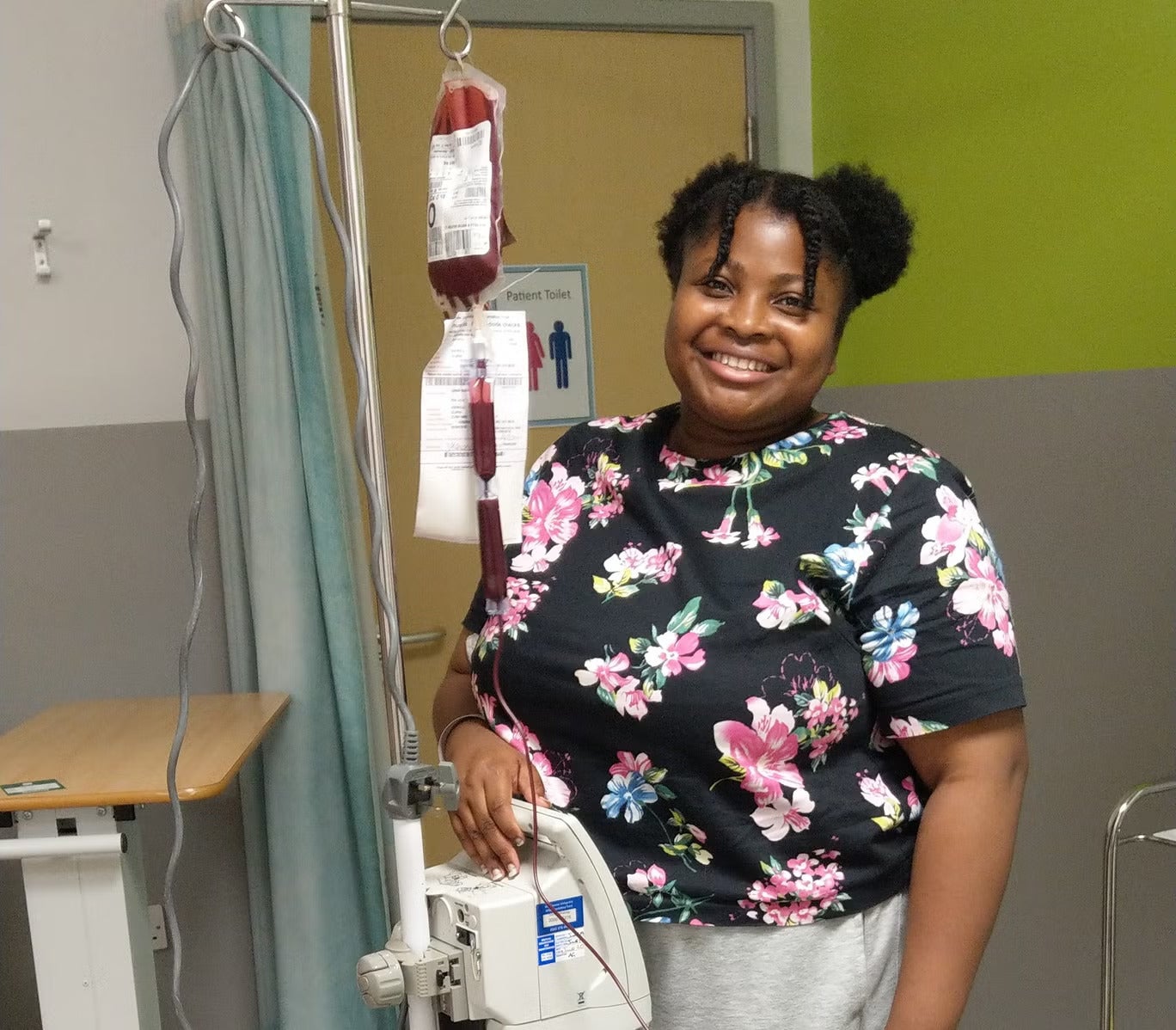
Speaking to The Independent in November 2022, sickle cell patient Sanah Shaikh, 33, described how much her life had improved since she began being treated with crizanlizumab in April of that year. The marketing professional said she had experienced fewer crises and the ones she had had could be managed at home with pain medication, rather than requiring hospital treatment.
“With this new treatment, I feel like I’ve been given an extra set of wings, to live with more courage and tenacity,” Ms Shaikh said.
Gloria, another patient, told of how “excited’ she felt about life after starting the new treatment, adding: “I can see the difference in my energy levels and behaviour too; it’s making me want to go out more, go out there, get things done and meet new people.”
Julian Beach, MHRA interim executive director of healthcare quality and access, told The Independent: “The... authorisation for sickle cell disease medicine Adakveo (crizanlizumab) has been revoked in the UK after a recent study failed to confirm its clinical benefits.
“This decision follows a careful review by the MHRA of the available evidence, which concluded that the potential benefits of the medicine did not outweigh its risks.
“Adakveo is being taken off the market in the UK and no new patients should be prescribed this medicine. Prescribers should contact patients currently on treatment with Adakveo to discuss alternative treatment options with them.
“We want to reassure UK patients using Adakveo that our review did not raise new safety concerns about the drug. If you have any questions about your treatment, please speak to your healthcare provider.”
NHS England declined to comment on this matter.
NICE is currently deciding whether it will approve the only other new sickle cell therapies – Casgevy and Voxelotor – for NHS rollout.



Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments