'Three-parent baby' fertility treatment could be available on NHS by next spring
Independent panel of experts recommend 'cautious adoption' of controversial therapy to treat mitochondrial disorders
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.A controversial "three-parent baby" treatment could be available on the NHS as early as next spring.
An independent panel of experts has recommended the "cautious adoption" of mitochondrial replacement therapy (MRT) for couples who carry potentially devastating inherited diseases.
The UK fertility regulator, the Human Fertilisation and Embryology Authority (HFEA), is now almost certain to give the final go-ahead for the treatments.
Scientists at the University of Newcastle, which has pioneered the treatments, say they already have women lined up for the therapy and are ready to apply for a licence to begin the procedures.
The team hopes to treat up to 25 women a year with NHS funding.
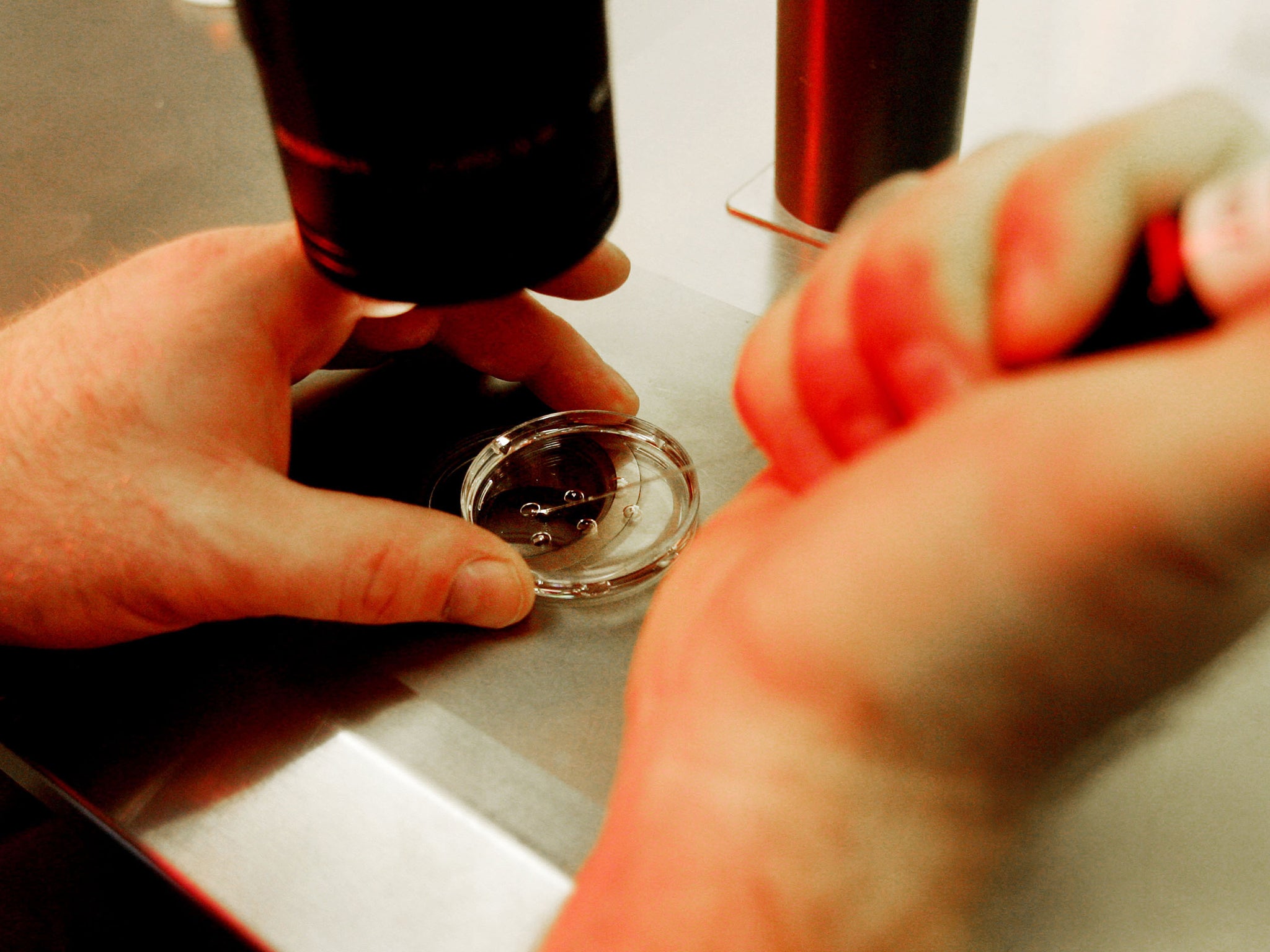
Babies born after MRT would effectively have three genetic parents. A tiny proportion of their DNA would come from their mother, father and a third person, an egg donor.
The therapy works by replacing the mitochondrial DNA in an embyro with healthy DNA from an egg donor to prevent the baby inheriting genetic diseases carried in this part of the cell.
Mitochondria only hold around 0.1 per cent of a person's DNA, which is always inherited from the mother and is responsible for producing the energy the rest of the cell needs to function.
It has no influence over individual characteristics such as appearance and personality and is separate from the DNA in the cell nucleus which house the vast majority of an individual's genes.
But when mitochondrial DNA (mtDNA) goes wrong, the results can be catastrophic.
Different mitochondrial disorders, such as Alpers, Leigh's disease, MELAS and MERRF, affect the body differently but they often mean parts of the body do not get the energy they need to function which can lead to muscle decay, blindness, organ failure and impaired physical and mental development.
For instance, Alpers disease is a neurological disorder which can cause seizures in even young children, means muscles do not develop properly and sufferers slowly lose the ability to think, speak or remember things. It can also lead to liver failure and blindness as the nerves controlling them decay.
In total mitochondrial diseases are believed to affect one in every 4,000 people.
Last year, the UK became the first country in the world to legalise the therapy after MPs and peers voted in favour.
The HFEA will conside the issue at a meeting on 15 December and will decide if clinics can make applications to offer the treatment.
It it approves the treatment, the first woman could undergo the procedure in as early as March or April next year.
Additional reporting by PA
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments