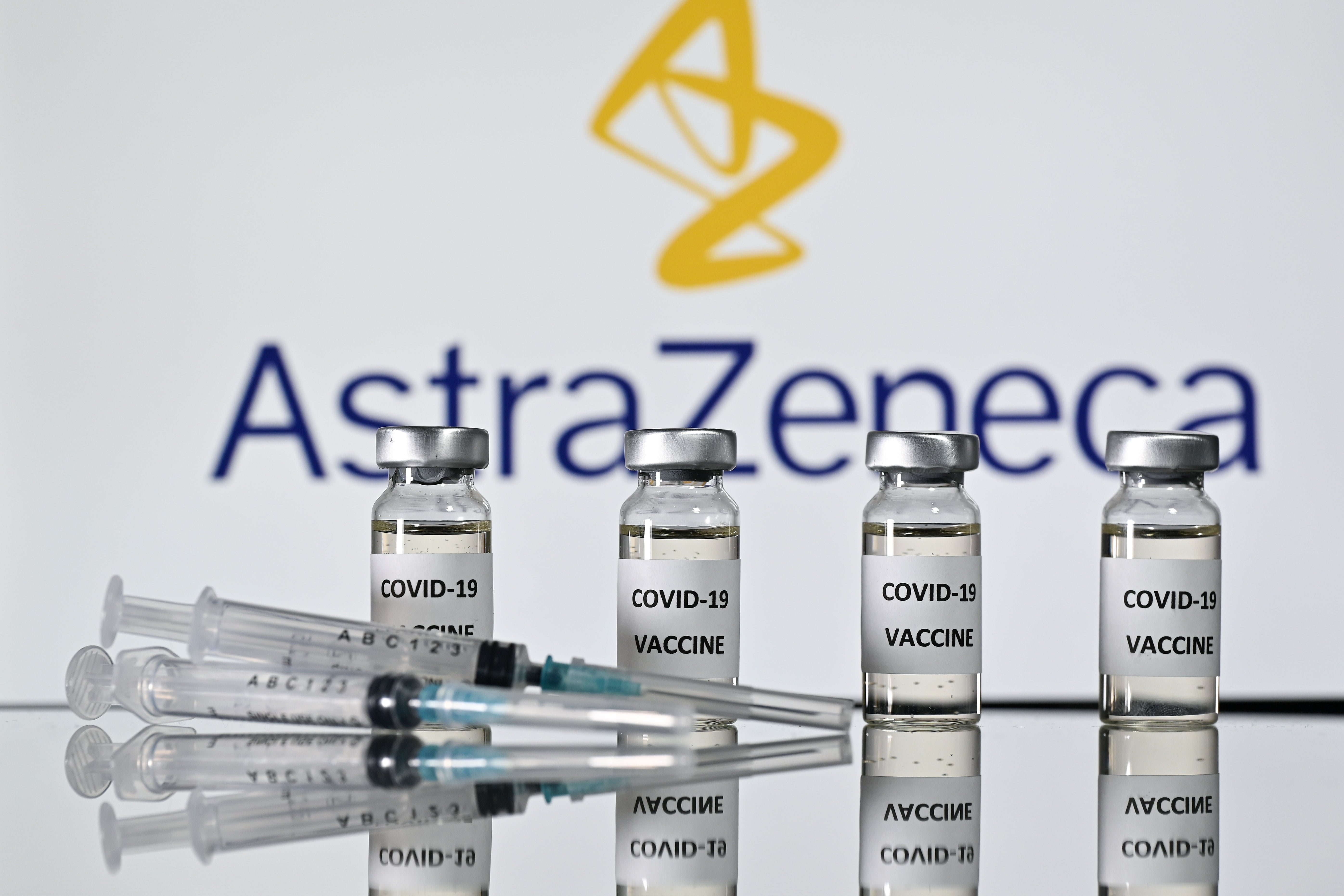
India likely to approve the AstraZeneca Covid vaccine by next week
India will become the first country to greenlight AstraZeneca
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.India is likely to soon join a growing list of countries that have started to roll out a vaccine for mass inoculation. It might give the go ahead to a Covid-19 vaccine developed by AstraZeneca and University of Oxford by next week, according to reports.
India’s leading drugmaker, the Serum Institute of India (SII), which asked for emergency approval of AstraZeneca on 7 December, has submitted the additional data asked of them to authorities.
Indian health officials are in direct contact with AstraZeneca and the University of Oxford and there are “strong indications", according to sources quoted by Reuters, that the jabs would be approved by next week.
India, the second-worst affected country in the pandemic, would become the first nation to give regulatory approval to British drugmaker’s AstraZeneca as trials for the vaccine continue.
SII, the Pune-based world’s biggest drug manufacturer, is collaborating with AstraZeneca to conduct late-stage trials of the vaccine and would produce and distribute its vaccine domestically.
“Serum is ready,” one of the sources told Reuters. “Initially, we may get around 50 million to 60 million doses.”
Serum Institute of India did not immediately respond to The Independent’s request for confirmation of this.
An expert panel will now examine the data and consider the proposal. The officials are still awaiting more details from Pfizer and Bharat Biotech.
On Tuesday, Dr V K Paul, who co-chairs the high-level committee on vaccine administration, said regulators have started the process of examining additional data.
“As everyone knows, there are three applications before the DGCI; they include Pfizer, Serum Institute and Bharat Biotech. Pfizer has not yet submitted data. The other two companies had presented the data and the regulator sought additional data. This is a normal process,” Mr Paul said.
India’s health minister Dr Harsh Vardhan also said on Sunday that approval to a vaccine could be given in January.
Last month, Prime Minister Narendra Modi visited the SII’s facility in Pune to be briefed on the process of vaccine production and distribution. The SII has already manufactured almost 450 million doses of the AstraZeneca shot and plans to make a total of 400 million doses by July.
Apart from AstraZeneca, Covaxin by Indian drugmaker Bharat Biotech has also applied for authorisation.
Another candidate by India, Zydus Cadila is also developing an anti-coronavirus vaccine with the name ZyCoV-D and started late-stage trials in August.


Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments