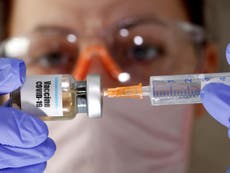
Peru halts trial of China Covid vaccine after patient suffers neurological problems
The government plans to roll out the vaccination process in the next three months

Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.The Peruvian government has suspended its trial of a major Chinese coronavirus vaccine candidate after a participant suffered a serious medical episode, even as the country claims it will begin rolling out jabs in the next three months.
The Peruvian minister of health Pilar Mazzetti said that the decision of the National Institute of Health (INS) to temporarily stop Sinopharm’s trial in the country was to evaluate the reason behind the adverse incident, which saw a participant experience neurological symptoms.
German Malaga, chief researcher at the local Cayetano Heredia University, which is involved in the study, said the volunteer had experienced decreased strength in his legs among other symptoms.
The authorities are trying to ascertain if the adverse incident is due to the vaccine or to other reasons. The vaccine was due to complete the first stage of the trials in the next few days.
The Sinopharm vaccine is not the only option available to Peru, and the government says it is in advanced stages of negotiations to acquire more than 26 million units of vaccine from Russia, China and the UK.
Ms Mazzetti noted that the most important thing for them is to have a vaccine that is safe for the population.
The Sinopharm vaccine is being developed by China National Pharmaceutical Group and is one among the several vaccines being developed by Chinese companies.
Last month, after an adverse incident, the health regulator of Brazil had suspended clinical trials of CoronaVac, another Covid-19 vaccine being developed by China.
According to the World Health Organisation, Peru has recorded over 980,000 coronavirus cases including 36,500 deaths.
On Sunday, the Peruvian government announced that it has almost finalised a deal for about 9.9 million doses of the Pfizer-BioNTech vaccine and the first batch could arrive as soon as this month. It said they also have an agreement with the WHO’s Covax Facility for 13.2 million units of vaccine, but they are only expected to come in the second half of 2021.
Additional reporting by agencies


Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments