Moderna ready to begin testing vaccine that fights South Africa strain
Several potential methods for addressing new coronavirus vaccines to be experimented with
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
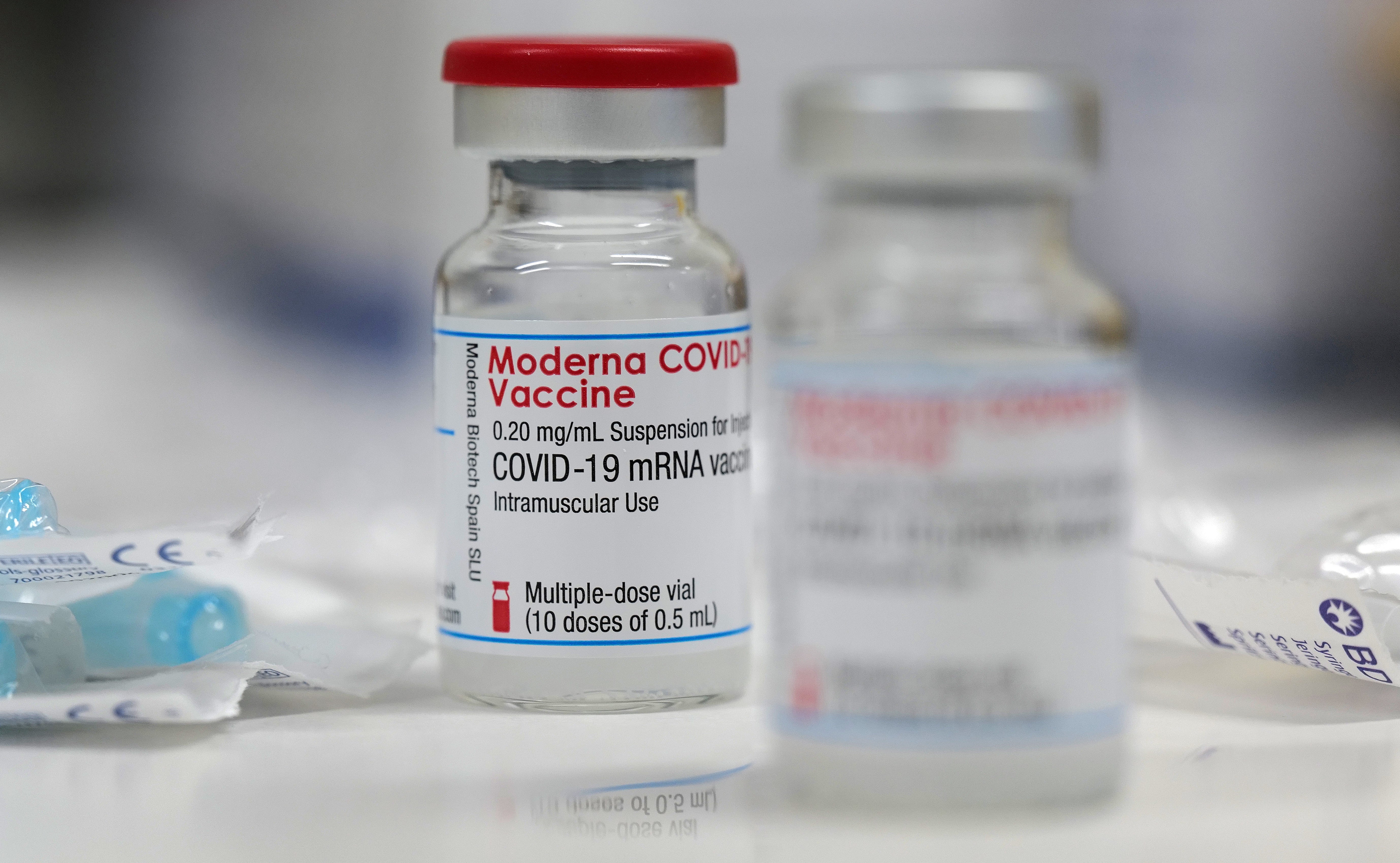
Your support makes all the difference.US biotech company Moderna has announced it is working with government scientists there to study an experimental booster shot specifically targeting the South African variant of Covid-19.
The firm has raised its global coronavirus vaccine production goal for this year by 100 million doses, it added.
It has produced raw material for a booster shot aimed at tackling the strain of Covid-19 that first emerged in South Africa, which may be more resistant to existing vaccines, said Moderna on Wednesday.
The new jab, code-named mRNA-1273.351, has been shipped to the US National Institutes of Health, which helped develop Moderna’s current vaccine, for additional study.
The development could make Moderna the first vaccine maker to have completed laboratory work of creating a jab that addresses new variants that emerged last year and have spread quickly throughout the world.
Moderna is experimenting with several potential methods of combating new variants of the virus, including an additional booster shot targeting the South African variant, a combined booster shot that mixes its current vaccine with the experimental shot, and an extra booster shot on top of its current two-dose vaccine.
It said it will also experiment with using its experimental shot and the combined shot as primary vaccinations against the virus, administering two-dose regimens for people who have not yet received a shot and have not been infected.
The South African variant of Covid-19 is spreading globally, with the US discovering its first case in January. It has since been identified in 14 states, according to official data.
Some studies have suggested it is more resistant to existing vaccines compared with other variants of the virus that emerged in South American and the UK.
Earlier this month, a study conducted by scientists at the University of Texas found that the Pfizer/BioNTech vaccine may still provide substantial protection against the variant - however, a separate study found the Oxford/AstraZeneca vaccine offers as little as 10 per cent protection.
South African halted the rollout of the Oxford/AstraZeneca jab following the small-scale trial, which was led by Professor Shabir Madhi from the University of Witwatersrand.
Moderna has also raised its expected vaccine production for 2021 to 700 million doses globally, and is exploring further improvements to its manufacturing process that could raise production to as many as a billion doses.
The company said it is also investing in additional manufacturing capacity that should bring its 2022 global production to around 1.4 billion doses, up from a previous projection of 1.2 billion.
It usually takes between six to nine months to develop a new manufacturing facility and additional time after that for regulatory inspection and to ramp up production.
About 60 million Moderna doses have been shipped so far, of which 55 million have gone to the US. The company said it is still in the midst of ramping up its supply chain for global shipments.
The announcement on Wednesday comes ahead of a planned investor call on Thursday morning.
Additional reporting by Reuters
Subscribe to Independent Premium to bookmark this article
Want to bookmark your favourite articles and stories to read or reference later? Start your Independent Premium subscription today.




Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments