FDA adds warning to breast implants in safety push
Patients will now have to complete a checklist showing they understand the potential risks, including memory loss, fatigue, joint pain, tiredness and cancer
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
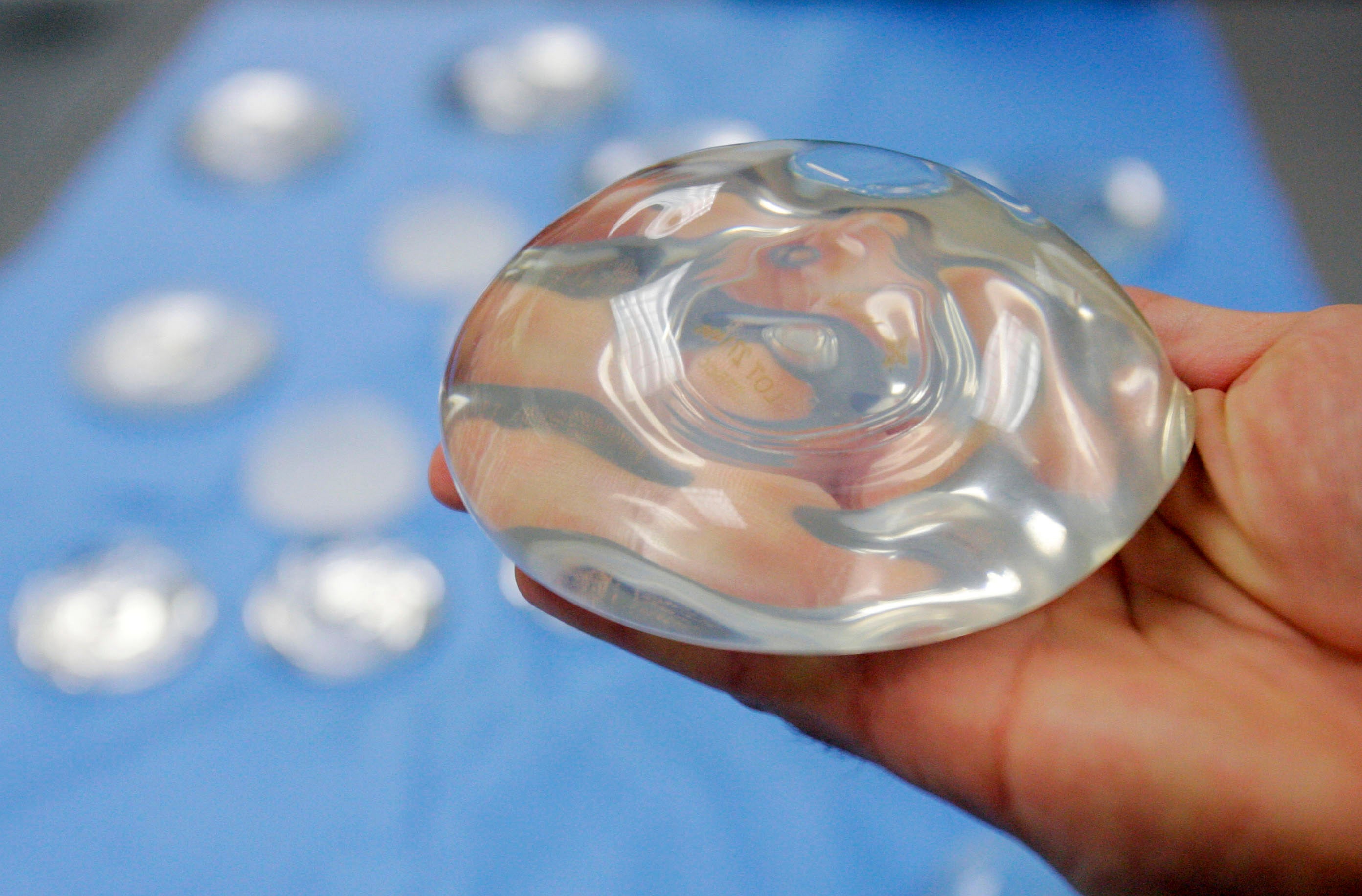
Your support makes all the difference.The US Food and Drug Administration has implemented additional safety requirements for patients looking to have breast implants.
New regulations mean that only healthcare providers will be able to sell and distribute breast implants, which must now come with a black box warning. In addition, those who offer the procedure must provide a standardised risk checklist to patients.
The regulations, released at the end of September, come following testimony at a 2019 hearing, in which women said their doctors did not properly advise them about the potential health complications of breast implants.
Now doctors, in addition to a face-to-face consultation, will have to ask future patients to complete a tick list to show they were properly informed about possible side-effects like potential memory loss, fatigue, joint pain, tiredness and brain fog, plus a condition dubbed “breast implant illness” – an implant-associated immune-system cancer named anaplastic large cell lymphoma (BIA-ALCL).
“Most people who are diagnosed with BIA-ALCL have their cancer develop eight to 10 years after breast-implant surgery,” states the Great Implant Cancer Advocates. Research is still being conducted into BIA-ALCL, but “it is believed that some breast implant material can cause lasting irritation inside the body and cause the immune system to overreact,” the Advocates write on their website.
Warnings will also tell potential patients that breast implants are temporary and not life-long, and chances of developing complications increase the longer a patient has them, in some cases surgery will be needed to address complications.
Breast augmentation is an elective procedure performed for those looking to increase breast size, change the shape of disfigured breasts or replace breast tissue that has been removed due to cancer or trauma – it is one of the top five performed cosmetic surgical procedures in the US.
Manufacturers will have 30 days from the end of September to display the updated device labelling on their products.
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments