Reports of unusual cancer linked to breast implants are rising, health agency warns
When detected early disease can usually be cured by surgery alone, but some women need more extensive treatment, including chemotherapy and radiation
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.More cases of an unusual cancer linked to breast implants have been reported to the Food and Drug Administration (FDA), the agency said.
The case count rose in the past year, to 414 cases from 359, the agency said in an update on its website. The number of deaths it has recorded, nine, has not changed from one year ago; a professional society of plastic surgeons is now reporting 16 related deaths.
The FDA’s figures include cases from the United States and other countries. The agency began publicly reporting on the problem in 2011 and some of the apparent rise in cases may be due to increased awareness and diagnosis.
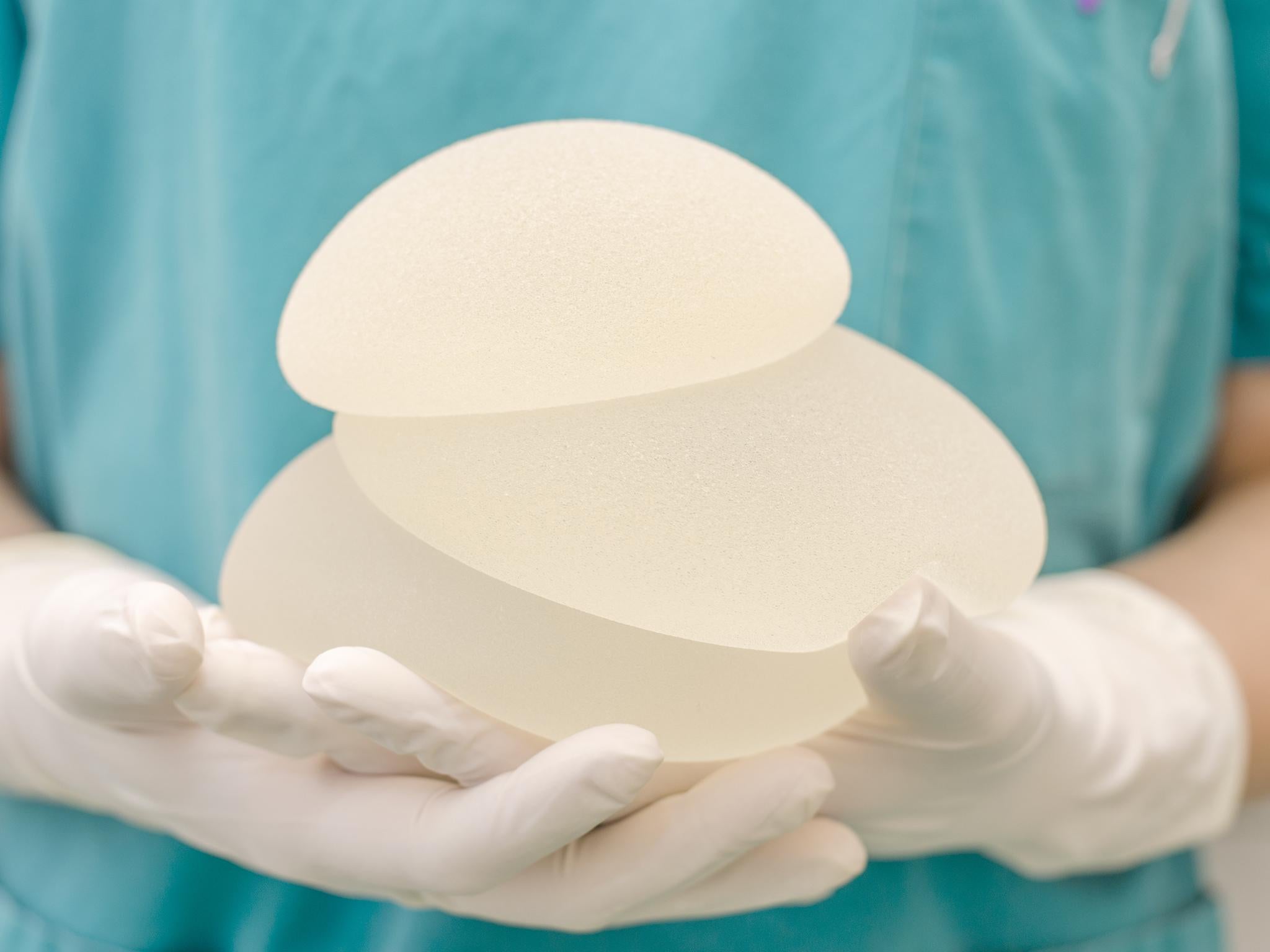
The disease is not breast cancer, but is a malignancy of the immune system called breast implant-associated anaplastic large-cell lymphoma. When detected early, it can usually be cured by surgery alone, by removing the implant and the capsule of scar tissue that forms around it. But some women have needed more extensive treatment, with chemotherapy and radiation and the disease can be fatal.
A major symptom is swelling around the implant, which has occurred from two to 28 years after the surgery, with a median of eight years. In women with no symptoms, there is no reason to remove implants or even to screen for the disease, the FDA said.
The lymphoma is more likely to occur in women with implants that have a textured coating, as opposed to a smooth cover, the agency said. No connection has been found between the disease and what is inside the implants – salt water or silicone. Nor is the lymphoma associated with breast cancer, as it is just as likely to occur in women who chose implants for cosmetic reasons, as in those who received them during reconstructive surgery after breast cancer.
Textured implants have roughened surfaces that may act as an irritant in some women, provoking inflammation that can lead to lymphoma, researchers say. Another theory is that the texturing might trap bacteria and that a chronic infection could also bring on the cancer.
Doctors and patients sometimes prefer textured implants because they are less likely to shift out of position than smooth ones, as tissue grows into the roughened surface and holds the implant in place. In the United States, most implants are smooth; textured ones are more popular in Europe.
The lymphoma “appears to currently develop exclusively in women with textured implants,” according to the American Society for Aesthetic Plastic Surgery.
But the FDA said it could not state conclusively that textured implants were the sole cause of the disease, because there was not complete information in every case on what type of implants were used. Implants often have to be replaced and if a woman with lymphoma has had both types of implants it may be hard to tell which is to blame.
A “consumer update” by the FDA on Wednesday describes the lymphoma, but makes no mention of texturing or its role in the disease. An agency spokeswoman, Stephanie Caccomo, said the FDA would “take another look at” the update, adding, “our goal is not to gloss over anything, but to be consumer friendly”.
Mark Marmur, a spokesman for Allergan, a major manufacturer of breast implants, said by email that the company was paying for research by outside investigators into causes of the lymphoma, working with plastic surgery societies on improved surgical techniques, adding information about the disease to its labelling and website and giving surgeons educational materials for patients.
He added that on 1 January, Allergan changed the warranty on its textured implant to cover treatment for Allergan patients who received a diagnosis of the implant-associated lymphoma on or after that date, regardless of the implantation date.
A request for comment to the other major manufacturer of breast implants, Mr Mentor, was not answered in time for publication.
The case count is uncertain, the agency said, noting that the FDA depends on voluntary reporting that “may contain incomplete, inaccurate, untimely, unverified or biased data”. Indeed, a registry called Profile, being compiled by professional societies in plastic surgery, is reporting higher figures – about 500 cases worldwide and 16 verified deaths, including five deaths in the United States.
AP
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments