More deaths, injuries linked to recalled eyedrops
U.S. officials are reporting two more deaths and additional cases of blindness linked to eyedrops tainted with a drug-resistant bacteria
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.U.S. officials are reporting two more deaths and additional cases of vision loss linked to eyedrops tainted with a drug-resistant bacteria.
The eyedrops from EzriCare and Delsam Phama were recalled in February and health authorities are continuing to track infections as they investigate the outbreak.
In the latest government tally, 68 people were diagnosed with infections from the bacteria, which has now caused a total of three deaths and eight cases of people losing their vision, the Centers for Disease Control and Prevention reported on Tuesday. That's up from one death and five cases of permanent vision loss reported last month.
The CDC said four people have undergone surgery to remove an eyeball due to the infections.
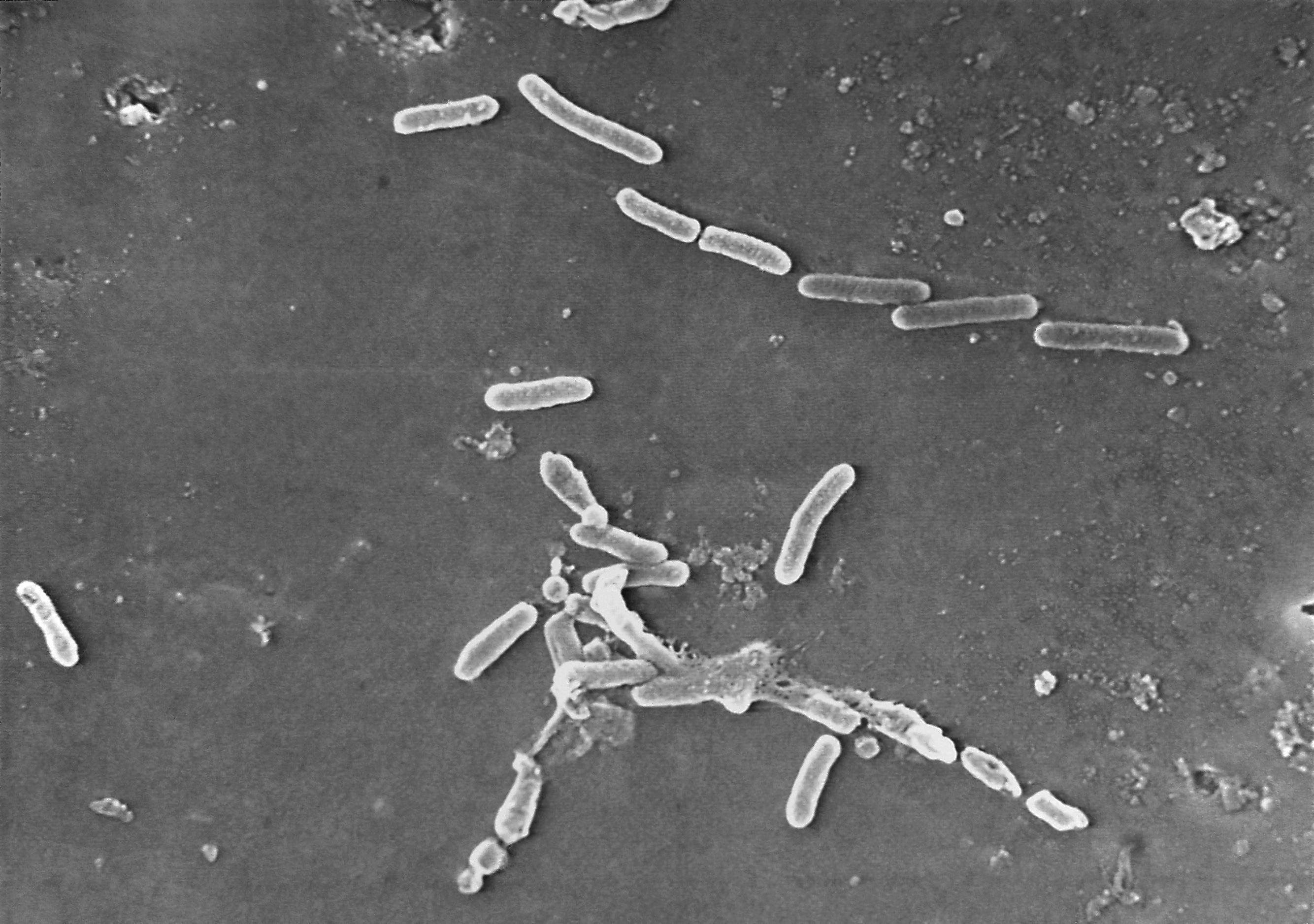
The outbreak is considered particularly worrisome because the bacteria driving it is resistant to standard antibiotics.
The CDC has now identified cases in 16 states, including California, New York, Illinois, Texas and Pennsylvania. Most of the cases have been linked to four regional clusters and Ezricare's drops are the only product used by patients in each of those groups.
The recalled drops were manufactured by Global Pharma Healthcare in India, where the bacteria — Pseudomonas aeruginosa — is commonly linked to outbreaks in hospitals. It can spread through contaminated hands or medical equipment.
___
The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute’s Science and Educational Media Group. The AP is solely responsible for all content.