VIRUS TODAY: Pfizer says vaccine can work against variants
New research suggests the COVID-19 vaccine made by Pfizer and BioNTech can still work against a mutated coronavirus
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.Here’s what’s happening Friday with the pandemic in the U.S.:
THREE THINGS TO KNOW TODAY
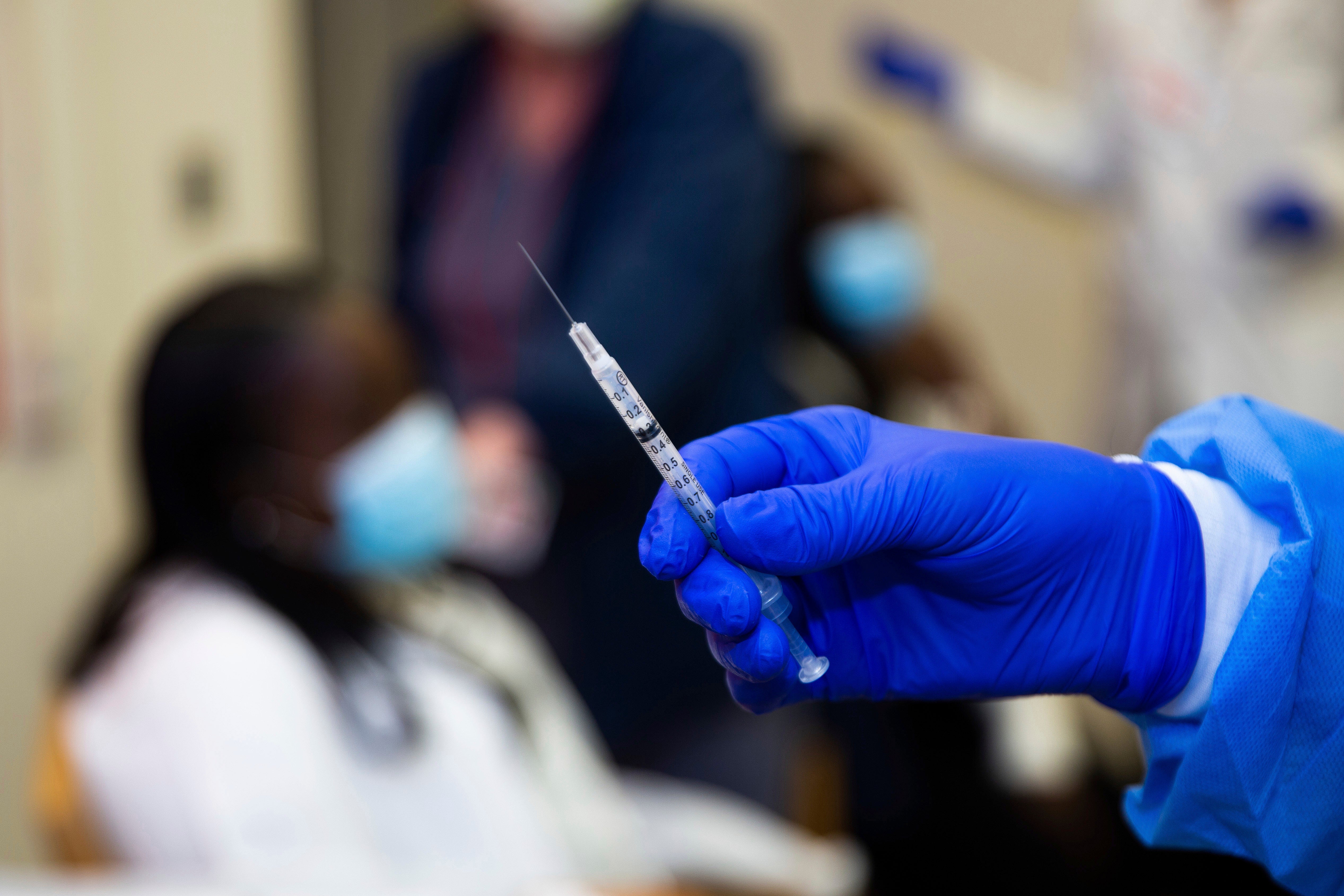
— New research suggests the COVID-19 vaccine made by Pfizer and BioNTech can still work against a mutated coronavirus. Two easier-to-spread new variants of the virus have the world on edge. One was discovered in Britain, the other in South Africa, but they share a common mutation. Pfizer researchers say lab tests show that mutation doesn’t block the vaccine. But more tests are needed to see if an additional mutation is cause for concern. The preliminary study was posted on an online research site late Thursday and has not been reviewed by other experts.
— Facing a massive surge in cases, California has been issuing waivers allowing hospitals to temporarily bypass the nation’s only strict nurse-to-patient ratios. Nurses say that being forced to take on more patients is pushing them to the brink of burnout and affecting patient care. At least 250 of about 400 hospitals in California have been granted 60-day waivers. They allow ICU nurses to care for three instead of two people and emergency room nurses to oversee six patients instead of three. Nurses in other states have demanded law-mandated ratios like those in California but so far have failed to get them.
— World Health Organization experts have issued recommendations saying that the interval between administration of two doses of the Pfizer-BioNTech vaccine can be extended to up to six weeks. WHO’s Strategic Advisory Group of Experts on immunization formally published its advice, saying an interval of 21 to 28 days between the doses is recommended. But the U.N. health agency also noted some countries face “exceptional circumstances of vaccine supply constraints combined with a high disease burden,” and some have been considering delaying the administration of a second dose as a way to broaden initial coverage.
THE NUMBERS: According to data through Jan. 7 from Johns Hopkins University, the seven-day rolling average for daily new deaths in the U.S. rose over the past two weeks from 2,595.1 on Dec. 24 to 2,764.1 on Jan. 7.
DEATH TOLL: The number of COVID-19-related deaths in the U.S. stands at 365,625.
QUOTABLE: “Just today we had two deaths on this unit. And that’s pretty much the norm. I usually see one to two every shift. Super sad. They fight every day, and they struggle to breathe every day even with tons of oxygen. And then you just see them die. They just die.” — Caroline Brandenburger, COVID-19 unit at St. Joseph Hospital in Orange, California
ICYMI: The nation’s second-largest city said it will keep using a coronavirus test that federal regulators warned may produce false results while Congress, which has used the same test, is seeking an alternative. The different responses Thursday followed a Food and Drug Administration alert to patients and health care providers that Curative’s test could particularly produce false negatives. Those faulty results pose the biggest risk from a health perspective because people who are erroneously told they don’t have the virus can unknowingly spread it to those around them. The California-based company said it was working to address the FDA’s concerns.
ON THE HORIZON: President-elect Joe Biden will be taking a new direction to speed release of coronavirus vaccines when he assumes office Jan. 20. His office said Friday Biden would curtail the current practice of holding back vaccine doses to guarantee that people who get their first shot can also get a required second inoculation three weeks later. Under the Trump administration’s approach, the government has been holding back a supply to guarantee that people can get a second shot, which provides maximum protection against COVID-19. After an initial glow of hope when vaccines were approved last month, the nation’s vaccination campaign has gotten off to a slow start.
___
Find AP’s full coverage of the coronavirus pandemic at https://apnews.com/hub/coronavirus-pandemic