Coronavirus: Government faces legal action over alleged ‘buy British’ policy in £80m antibody test contracts
Lawyers claim other companies were unlawfully blocked from bidding for work
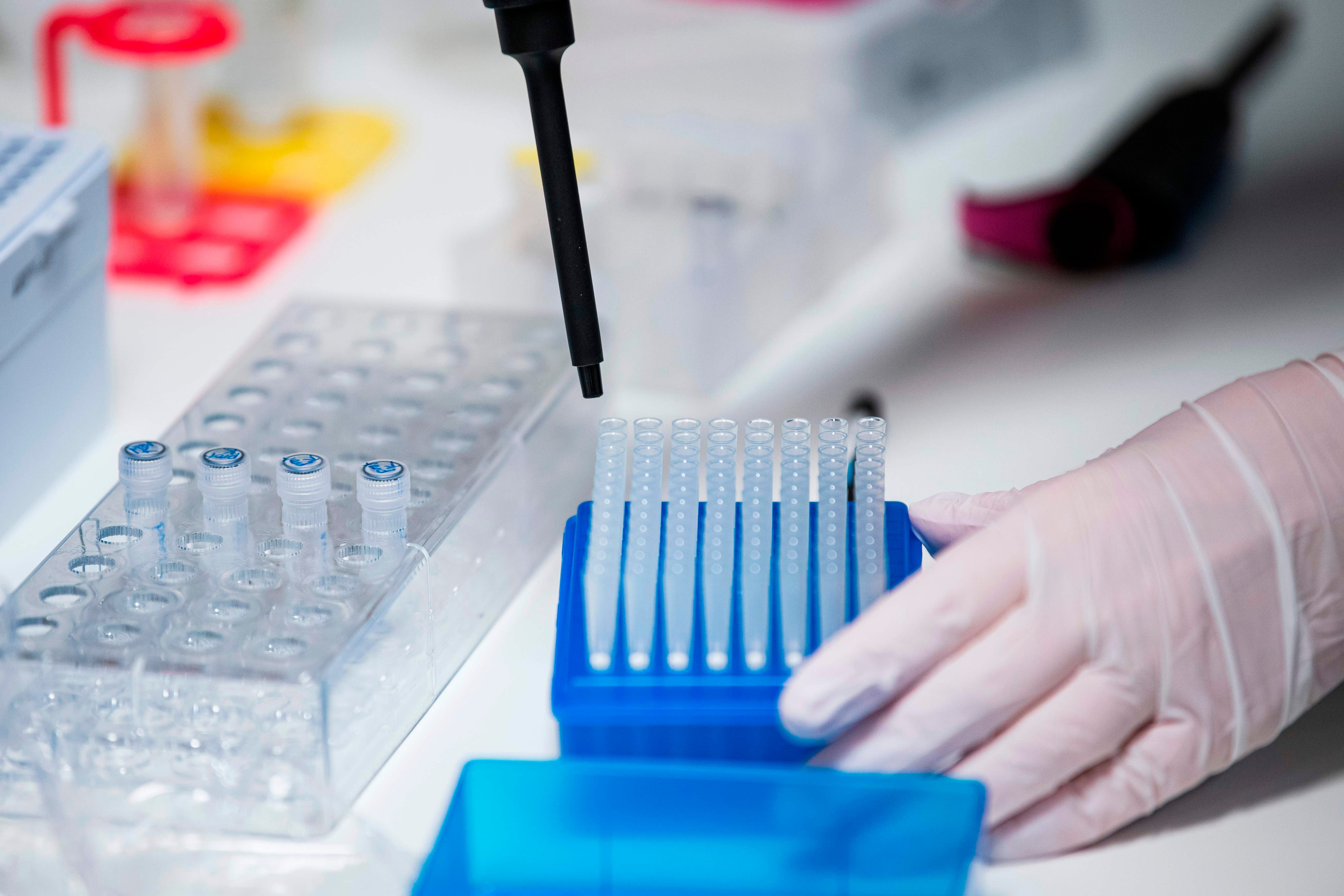
Your support helps us to tell the story
This election is still a dead heat, according to most polls. In a fight with such wafer-thin margins, we need reporters on the ground talking to the people Trump and Harris are courting. Your support allows us to keep sending journalists to the story.
The Independent is trusted by 27 million Americans from across the entire political spectrum every month. Unlike many other quality news outlets, we choose not to lock you out of our reporting and analysis with paywalls. But quality journalism must still be paid for.
Help us keep bring these critical stories to light. Your support makes all the difference.
The government is facing legal action over allegations it operated an illegal “buy British” policy when it awarded an £80m-plus contract to a UK company to provide coronavirus antibody tests.
Lawyers from the Good Law Project argue that other companies with a better track record in developing tests were not given the opportunity to bid for the contracts granted without competition to Abingdon Health by the Department of Health and Social Care (DHSC).
The deals were struck in the wake of the failed purchase in March of 3.5m Chinese antibody tests which turned out not to be useable.
In a legal document seeking judicial review of the decision, lawyers state that AH was “a small company with no existing rapid antibody test product or any track record demonstrating any unique or even exceptional capability to develop such a product”.
The tests it produced were insufficiently sensitive for their intended use to allow the issuing of “immunity passports” to people who had had Covid-19 and recovered, and were eventually used instead for surveillance programmes.
Although AH claimed 99 per cent accuracy for the finger-prick test, Public Health England found a real-world accuracy rate of around 85 per cent.
This means that if individuals were tested to see if they had been infected with Covid-19 and recovered, around one in five positive results would be false positives.
The PHE study warned: “Apart from limited surveillance to estimate the proportion of a population that has been infected, widespread use of this assay in any other role could risk considerable harm.”
In its submission, the Good Law Project stated that the award of the contracts in June and August this year without any advertisements or open competition was unlawful because at this stage of the pandemic there was “no unforeseeable extreme urgency” permitting health secretary Matt Hancock to bypass fair competition rules.
The DHSC was “unlawfully influenced” by national preference, because it wanted the contracts to go to a British company like AH, as well as by pre-existing relationships between the firm and the government, said the document.
And it said that DHSC had a “direct financial interest” in the future success of AH and its tests, as it took a slice of revenue from them.
“There were a number of economic operators active in the market who had already developed rapid antibody test products and/or had an established track record of developing such products,” said the submission.
“None of these economic operators was afforded any opportunity to be awarded the contracts or demonstrate the capability to meet the defendant’s requirements.”
The government announced in April that a new Rapid Test Consortium involving AH and Oxford University had launched to design and develop “a new antibody test to determine whether people have developed immunity after contracting the virus”.
Contract documents in June and August confirmed that the consortium was developing tests for the so-called Pillar 3 of the government’s national testing strategy, for mass antibody checks to see if people had immunity to coronavirus.
But an announcement in October that the government was buying 1 million tests said instead that they would be used in the less urgent Pillar 4 surveillance programme, which aims to learn more generally about the disease and its spread.
The DHSC said it was unable to comment on an ongoing legal case.
Subscribe to Independent Premium to bookmark this article
Want to bookmark your favourite articles and stories to read or reference later? Start your Independent Premium subscription today.




Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments