Cosmetic surgery firms face ban on giving free advice
Your support helps us to tell the story
This election is still a dead heat, according to most polls. In a fight with such wafer-thin margins, we need reporters on the ground talking to the people Trump and Harris are courting. Your support allows us to keep sending journalists to the story.
The Independent is trusted by 27 million Americans from across the entire political spectrum every month. Unlike many other quality news outlets, we choose not to lock you out of our reporting and analysis with paywalls. But quality journalism must still be paid for.
Help us keep bring these critical stories to light. Your support makes all the difference.
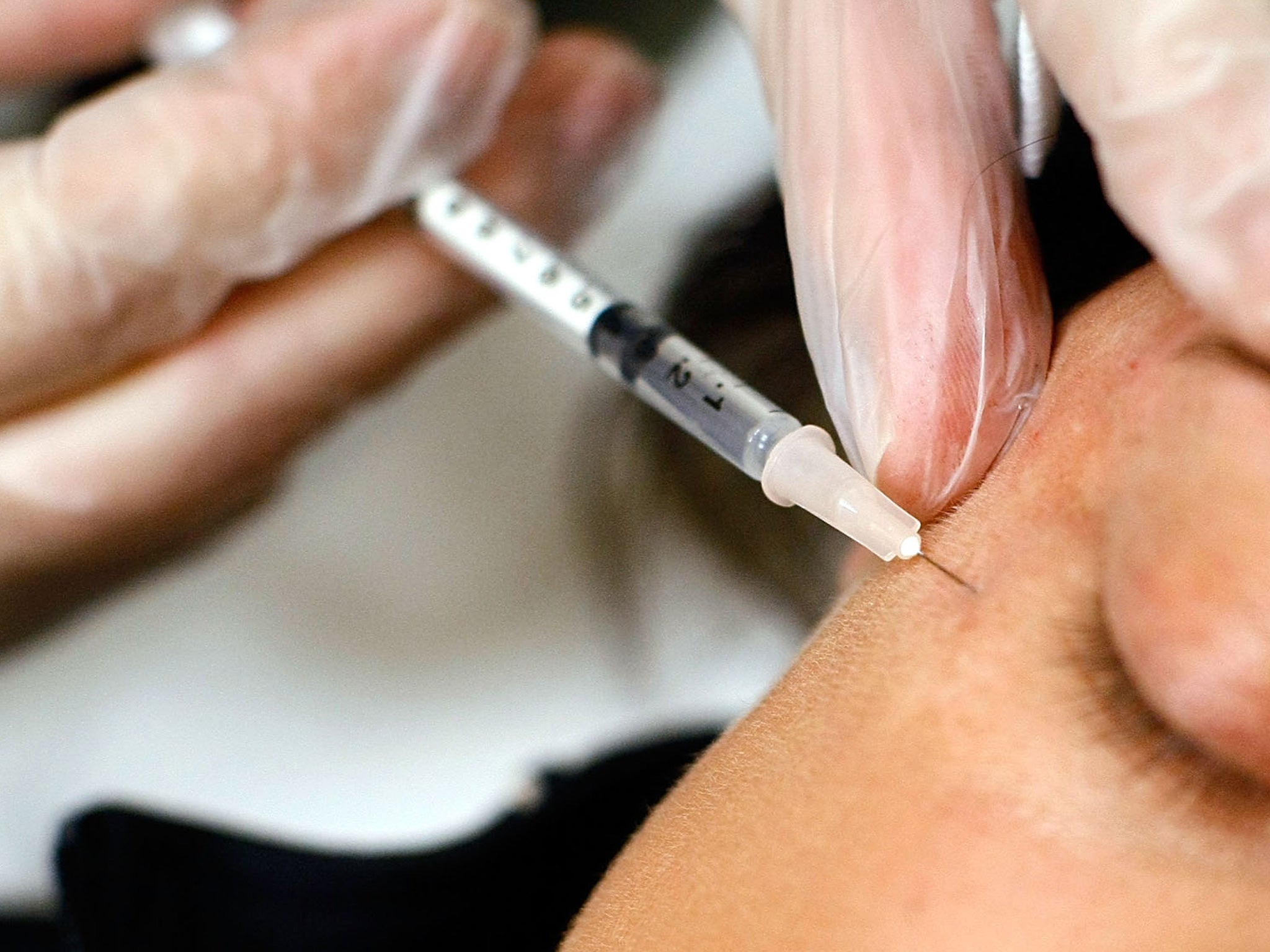
Cosmetic surgery companies face being banned from offering free medical consultations to prospective patients and may be required to obtain a two-stage written consent in a clampdown on the industry in the wake of the PIP breast implant scandal.
As part of a review into the cosmetic surgery industry, the Government is also considering banning companies from offering two-for-one surgical procedures and time-limited deals. Practitioners would also be expected to provide better information to patients, including photos of expected bruising and scarring, and more detail on the risks associated with surgery.
Publishing the public response to its consultation on toughening up the laws that govern cosmetic surgery, Sir Bruce Keogh, the medical director of the NHS, said it was clear that the current regulatory framework didn't do enough to support consumer rights or patient safety.
"It's not always acknowledged that people undergoing cosmetic interventions are not only consumers but also patients," he said. "They are taking decisions about medical procedures that can have a profound impact on their health. The supply and demand for procedures has outgrown the existing legislation."
The review was requested by Health Secretary Andrew Lansley last January after concerns were raised following the public outcry over faulty PIP breast implants.
Around 40,000 women in the UK received implants manufactured by the now-closed French company Poly Implant Prostheses, mostly in private UK clinics. The implants were filled with non-medical grade silicone intended for use in mattresses.
The Government is due to publish its final recommendations in March. But in an indication of what areas are likely to be included it today publishes the recommendations which had most public support and that were being actively considered for further regulation. These include banning free consultations to avoid people feeling obliged to have procedures.
The report was welcomed by the British Association of Aesthetic Plastic Surgeons (BAAPS).
Subscribe to Independent Premium to bookmark this article
Want to bookmark your favourite articles and stories to read or reference later? Start your Independent Premium subscription today.
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments