Up to 10,000 Britons could take part in cancer vaccine trials
Participants could receive groundbreaking treatment after the Government signed an agreement with a leading pharmaceutical company.
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
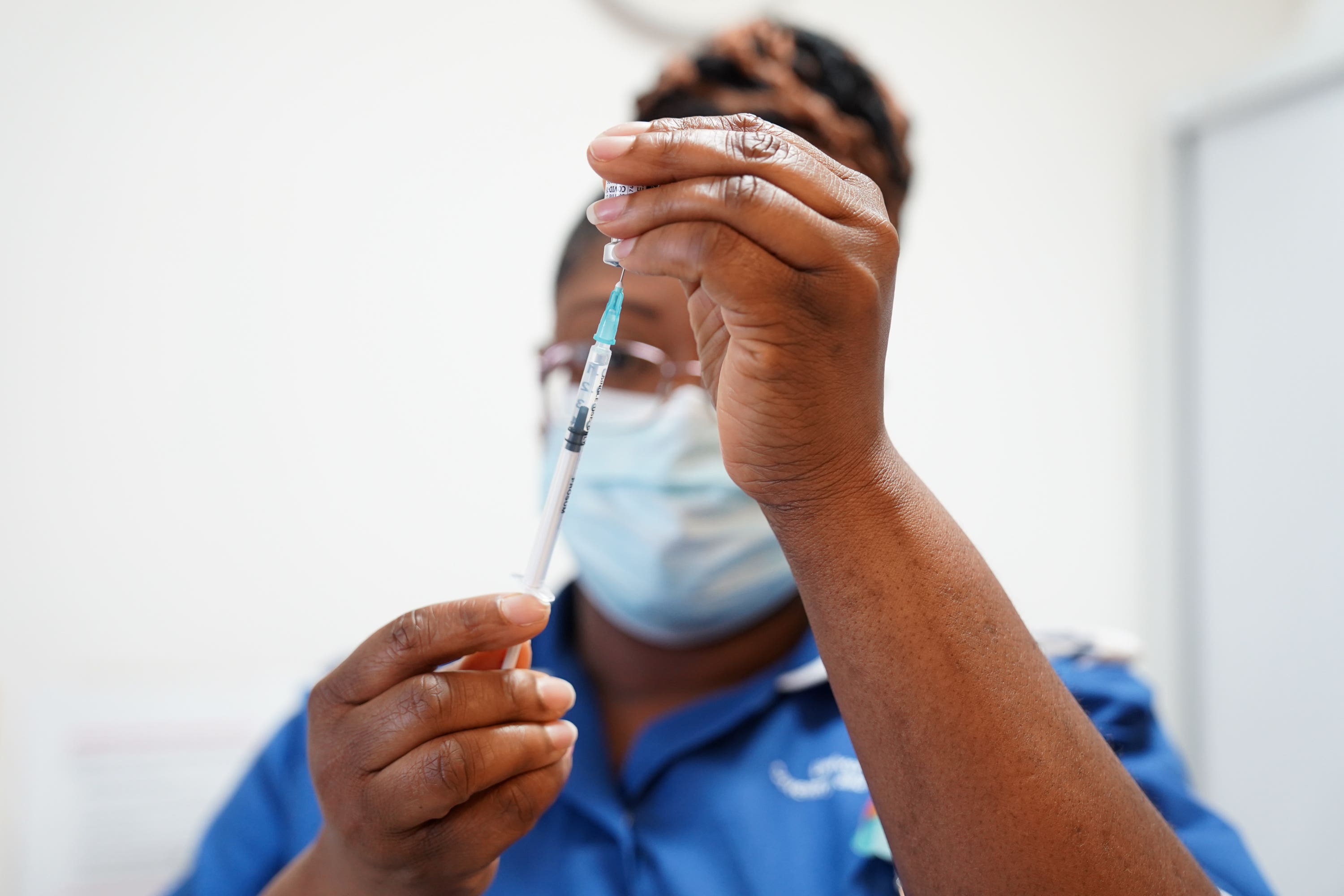
Your support makes all the difference.Up to 10,000 Britons are set to take part in clinical trials for personalised cancer vaccines by 2030 after the Government signed an agreement with a leading pharmaceutical firm.
Patients will be given precision immunotherapies which work by stimulating the immune system to recognise and eliminate cancer cells.
The announcement comes after the Government signed an agreement with German-based company BioNTech – which previously developed a coronavirus vaccine with Pfizer in less than a year.
The partnership, which builds on a memorandum of understanding the Government and the pharmaceutical firm signed in January, will see BioNTech set up new laboratories in Cambridge which are expected to employ more than 70 top scientists.
Personalised cancer vaccines have the potential to completely revolutionise the way we treat this cruel disease
Prime Minister Rishi Sunak said: “This landmark new agreement takes us one step closer to delivering life-saving new cancer treatments for thousands of patients right across the country.
“The UK is a global leader in life sciences – helping to create thousands of highly skilled jobs and pioneering research – and it is testament to this success that BioNTech have chosen to make this significant investment here today.
“Personalised cancer vaccines have the potential to completely revolutionise the way we treat this cruel disease and it is hugely welcome that, thanks to today’s announcement, clinical trials will be rolled out widely”.
Trials will focus on personalised mRNA-based cancer immunotherapies which seek to activate a patient’s immune system.
The technology is similar to that used in the Pfizer/BioNTech coronavirus vaccine.
They can either be designed to target shared abnormalities in a specific type of cancer or tailored to an individual’s tumour.
A new Cancer Vaccine Launch Pad (CVLP) will create a database to help quickly identify cancer patients who could be eligible for potential trials.
Most participants are not expected to enrol before 2026 and they will have to consent.
The partnership will seek to help people with early and late-stage cancers and, if successfully developed, cancer vaccines could become part of standard care.
Health Secretary Steve Barclay said: “This partnership is a huge step forward in the fight against cancer.
“I’m excited by the potential these trials have to both treat patients with cancer and those who have had it to stop it returning.
“This further demonstrates that the UK is an attractive location for innovative companies to invest and pioneer cutting-edge treatments for our patients.”
Amanda Pritchard, chief executive of the NHS said: “The NHS will not stop in its efforts to pioneer new treatments that could be life-changing for future generations.
“This is why we are developing our very first Cancer Vaccine Launch Pad, enabling us to identify thousands of NHS patients suitable for cancer vaccine trials – giving them the earliest possible access to cutting-edge technology that has the potential to change cancer care forever.
“Thanks to advances in treatment and care alongside NHS awareness campaigns, cancer survival is at an all-time high, but the potential to stop cancer from returning is truly remarkable.”
Professor Ugur Sahin MD, chief executive and co-founder of BioNTech said: “We are truly honoured to be an integral part of this landmark partnership, alongside the UK government, NHS England, Genomics England and the National Institute for Health and Care Research.
“The United Kingdom’s expertise in genomic analyses in cancer patients is a critical component of our shared endeavour to make mRNA-based and precision cancer immunotherapies widely accessible through clinical trials.
“If successful, this collaboration has the potential to improve outcomes for patients with cancer not just in the UK, but also worldwide.”
On Thursday, the Health Secretary will convene a roundtable with NHS leaders and health experts to discuss how technology can drive innovation throughout the service.