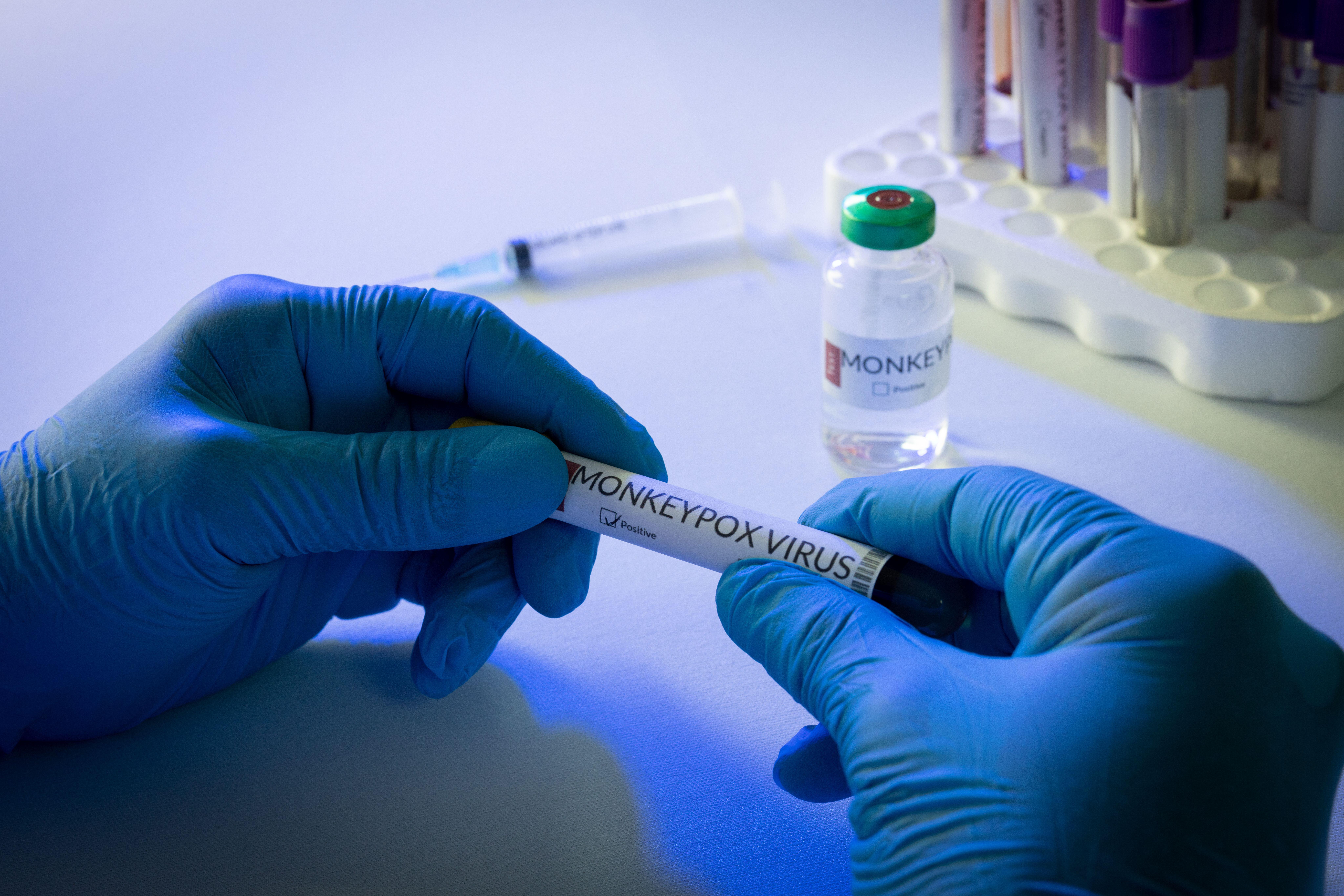
Trial launched to find treatment for monkeypox
The Platinum trial will be the first randomised controlled trial of a treatment for monkeypox in humans in the world.
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.British scientists have become the first in the world to launch a clinical trial to asses a treatment of monkeypox in humans.
Academics from the University of Oxford who were behind the Covid Recovery trial want to assess whether an antiviral drug can help alleviate symptoms among those infected.
The researchers are hoping to recruit 500 people from around the UK to join the trial to test whether Tecovirimat – also known as Tpoxx – can help reduce the amount of time people are sick.
The drug, which was created to treat smallpox, works by preventing the virus from leaving the infected cell, which stops the spread of the virus within the body.
Health officials in the UK licenced the drug earlier this year based on results from initial studies in animals and safety evidence from human volunteers.
It is currently offered to patients with severe complications who are being treated in hospital.
But to date there have been no clinical trials to confirm whether Tpoxx can help monkeypox patients recover from the disease so researchers who were behind the Recovery trial during the Covid-19 pandemic have launched a trial to test the drug in humans.
The Platinum trial will be the first randomised controlled trial of a treatment for monkeypox in humans in the world.
This means that half of the participants – who will have a confirmed diagnosis of monkeypox but not be poorly enough to need hospital care – will be given Tpoxx, while the other half will be given a placebo, or dummy drug.
Most patients with monkeypox will be eligible to enrol to the trial, which will involve them taking the medication, or the placebo, twice a day for 14 days.
They will be followed up for a month to assess whether taking the drug reduces symptoms – including the length of time people have painful skin lesions – or whether it could reduce the amount of time people stay in isolation.
This study is a very important next step towards looking at treatments for monkeypox for those being outside of hospital
Researchers will also examine whether the drugs can reduce the amount of time people test positive for the virus.
While vaccines developed for smallpox may reduce the risk of catching monkeypox, there are currently no proven therapeutics to speed recovery in those who develop the disease.
The study, commissioned and funded by the National Institute for Health and Care Research (NIHR), saw its first participant recruited on Friday.
Sir Peter Horby, professor of emerging infections and global health at the University of Oxford, said: “Monkeypox is a distressing and sometimes dangerous infection.
“For the benefit of current and future patients worldwide who have been diagnosed with monkeypox, we need definitive evidence that tecovirimat is safe and effective.
“Although the early data on tecovirimat are promising, only a randomised clinical trial will provide the level of evidence we need to treat patients with confidence. Platinum will provide that evidence.”
Professor Lucy Chappell, chief executive of the NIHR and chief scientific adviser at the Department of Health and Social Care, said: “This study is a very important next step towards looking at treatments for monkeypox for those being outside of hospital.
“It’s crucial that we invest in developing, refining and evaluating treatments for this disease.”
Public health minister Maggie Throup said: “This government-funded study is an important step to finding a treatment which can help speed up the recovery of those who have monkeypox.
“Vaccines remain our best defence against the spread of monkeypox – we urge all those eligible to come forward when contacted, and report any symptoms to NHS 111”.
On Monday the UK Health Security Agency launched a pilot offering eligible patients smaller doses of monkeypox vaccine, amid global shortages of the jab.
The Welsh Government has also confirmed that it will pilot a “fractional dosing” approach to monkeypox vaccination.
Since the start of the outbreak there have been more than 3,000 UK cases, with most of these identified in England.