Stem cell trial cures blindness for many patients - with no side effects
Phase one surgery trial to cure macular degeneration hailed as a success
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.A pioneering treatment for progressive blindness has been proved safe three years after patients were injected with stem cells derived from human embryos, a study has found.
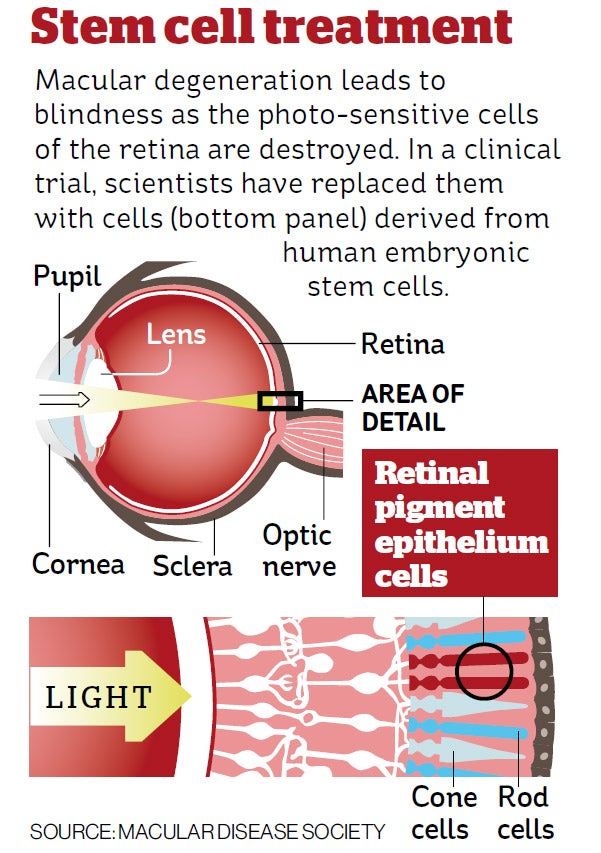
More than half of the patients with macular degeneration – where the eye’s light-sensitive cells are progressively destroyed – experienced a significant improvement in their eyesight, but none showed any adverse effects due directly to the transplant of the embryonic cells, scientists said.
Doctors injected the stem cells into the eyes of 18 patients – nine with Stargardt’s macular dystrophy and nine with dry, age-related macular degeneration – with the ultimate aim of repairing damaged photoreceptors in the retina at the back of the eye.
However, the more immediate goal of the phase one clinical trial was to test the safety and tolerability of the operation, which involved injections with three different doses – 50,000, 100,000 and 150,000 cells – said Robert Lanza, chief scientific officer of Advanced Cell Technology in Marlborough, Massachusetts.
No adverse safety issues were found during the three years that the 18 patients were monitored. There was also evidence that the injected cells had survived, and had contributed to an improvement in the eyesight of some patients, Dr Lanza said.
“About half of the patients had an improvement in visual acuity of three lines or more, which corresponds to a doubling of the visual angle, and is generally accepted as clinically significant,” Dr Lanza said.
“To understand what this means, let’s assume you’re six feet tall. If a patient can now see you after treatment, then before treatment you would have to have been 12 feet high – taller than a flagpole – in order for them to see you,” he said.
One 75-year-old rancher from Kansas, who was effectively blind in one eye before the treatment, improved to such an extent that he was able to ride a horse again. Other patients reported that they could use their computers or could go shopping, Dr Lanza said.
Follow-up testing found that 10 out of the 18 patients experienced substantial improvements in how well they could see, according to the study published in the journal The Lancet.
Further clinical trials will be carried out to test the efficacy of the treatment.
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments