Genetically testing human embryos: why the designer baby debate is so heated
The issue is fraught with ethical and legal complexities – but also at the heart of the matter are contrasting opinions about what makes an acceptable clinical trial
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.Screening embryos for genetic abnormalities in IVF was first successfully performed in 1989, resulting in the birth of the Munday twins. A test to screen for a specific genetic abnormality was later developed, choosing embryos with the correct number of chromosomes and discarding those with too many or too few. This test is widely used in fertility clinics to increase the chance of live birth and a healthy baby. Whether it should be performed at all, however, is hotly debated.
Both egg and sperm cells normally have 23 chromosomes, and after fertilisation a single-celled embryo with 46 chromosomes is formed. A cell with 46 chromosomes is known as “euploid”, and an abnormal cell, with fewer or more chromosomes, is known as “aneuploid”.
Aneuploidy can lead to congenital abnormalities (such as Down syndrome), pregnancy loss, infertility or IVF not resulting in pregnancy.
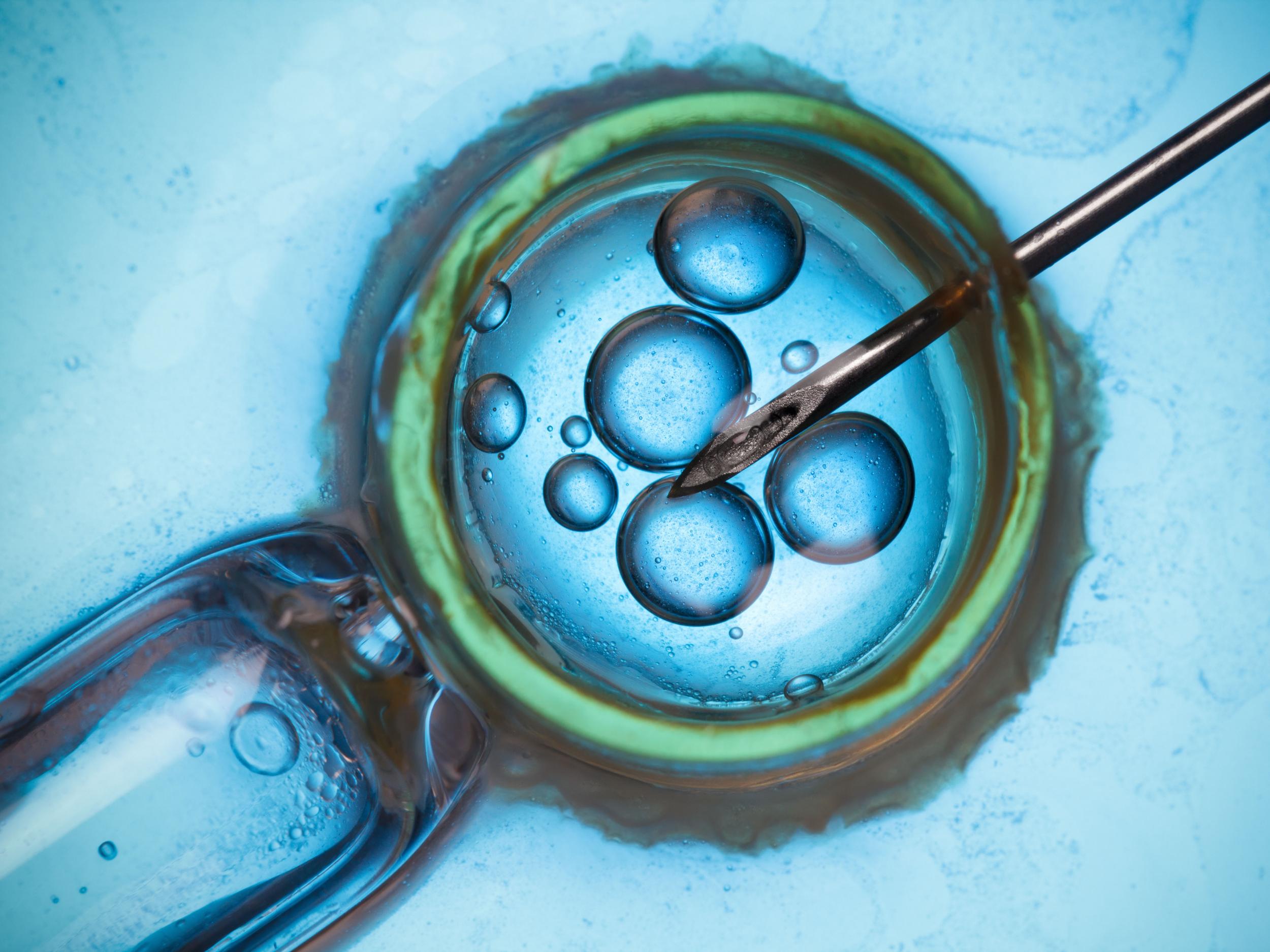
Embryonic cells continue dividing after fertilisation and, after five or six days, there are about 100 cells, each with 46 chromosomes – if the cells are normal. One group of cells will become a baby and the other the placenta. At this stage a few cells that would later form the placenta can be removed by an embryologist for testing. The test is known as preimplantation genetic testing for aneuploidy, or PGT-A.
Trial by error
By only implanting euploid embryos, PGT-A should improve a woman’s chances of becoming pregnant. However, the first clinical trials to evaluate the procedure showed the reverse to be true, and authors of those trials remain fierce opponents of the test. But parents who have benefited from PGT-A, and many fertility experts, are enthusiastic advocates. A lively debate has persisted ever since.
Early versions of PGT-A used fluorescent probes to identify specific chromosomes three days after fertilisation, when the embryo has just eight cells. However, these methods were prone to misdiagnosis and didn’t look at every chromosome in the embryo. So abnormal aneuploid cells could be missed and normal embryos could be mistakenly classed as aneuploid, which partly explains the unfavourable clinical trial results.
As well as changing the embryo biopsy time to five or six days after fertilisation, more accurate and sensitive tests were developed to analyse all chromosomes simultaneously. These new approaches – demonstrated in several clinical trials, observational studies and large national data sets – have improved IVF results, increased pregnancy and birth rates, and reduced miscarriage. As a result, many fertility experts now believe PGT-A can be beneficial, using the right methods, in the right hands and with the right patients.
However, opponents of PGT-A argue there is still not enough high-quality evidence to justify PGT-A. They argue that PGT-A should only be used after at least one “properly designed” favourable clinical trial has been published, as they consider the previous trials, showing PGT-A to be of benefit, to be biased, poorly designed and too small.
The debate continues as PGT-A advocates argue there is already enough evidence to support PGT-A and that further clinical trials are unnecessary and too expensive. Also, they argue that many widely used IVF procedures, such as intracytoplasmic sperm injection (ICSI) – where a sperm is directly injected into an egg and fertilised before implantation – were never subjected to clinical trials as their benefits were immediately obvious.
At the core of the debate are contrasting opinions of what constitutes a well designed, well conducted clinical trial.
Aside from clinical trial evidence, there are other considerations about the cost of not providing PGT-A – in financial, medical, legal and ethical terms. For instance, ethically, what would be the harm to patients – medical and psychological – if they had an aneuploid pregnancy, assuming PGT-A wasn’t on offer?
Mosaicism
Another complicating factor is “mosaic” embryos, that is embryos containing both euploid and aneuploid cells. The latest technology, called next generation sequencing, is so sensitive it readily detects mosaic embryos. Some fertility experts consider this a benefit, as most mosaic embryos, if transferred, will not result in pregnancy. Others worry that, since healthy babies have been born following mosaic embryo transfer, many mosaic embryos could be unnecessarily discarded if they are classed as “abnormal”.
We need more research to understand how chromosomal abnormalities arise in embryos, mosaic or otherwise, and how to prevent them. One new diagnostic approach involves testing the fluid in which the embryo develops, rather than removing cells from the embryo. This fluid is thought to contain DNA from the embryo, and early results are promising.
PGT-A can be improved, but it will never be perfect – no test is. The disagreement between PGT-A opponents and advocates is whether this test is good enough now. Unfortunately, when new evidence does emerge, it always seems to further polarise opinion rather than leading to clarity and resolution.
Genetic counsellors are trained to demystify the complexities of issues, such as mosaicism, and explain to patients what the transfer of a mosaic embryo might mean for them. Fertility experts may continue debating the pros and cons of PGT-A, but patients can only make an informed choice if provided with all of the available evidence.
Darren Griffin is professor of genetics at the University of Kent; Alan Thornhill is honorary professor of reproductive genetics at the University of Kent; Cagrı Ogurbut is a PhD candidate at Yıldız Technical University. This article first appeared on The Conversation (theconversation.com)
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments