Novavax vaccine trialists to be offered two extra Pfizer jabs to allow them to travel
Individuals who received vaccines yet to be approved have been left in limbo, unable to receive authorised jabs or travel abroad
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
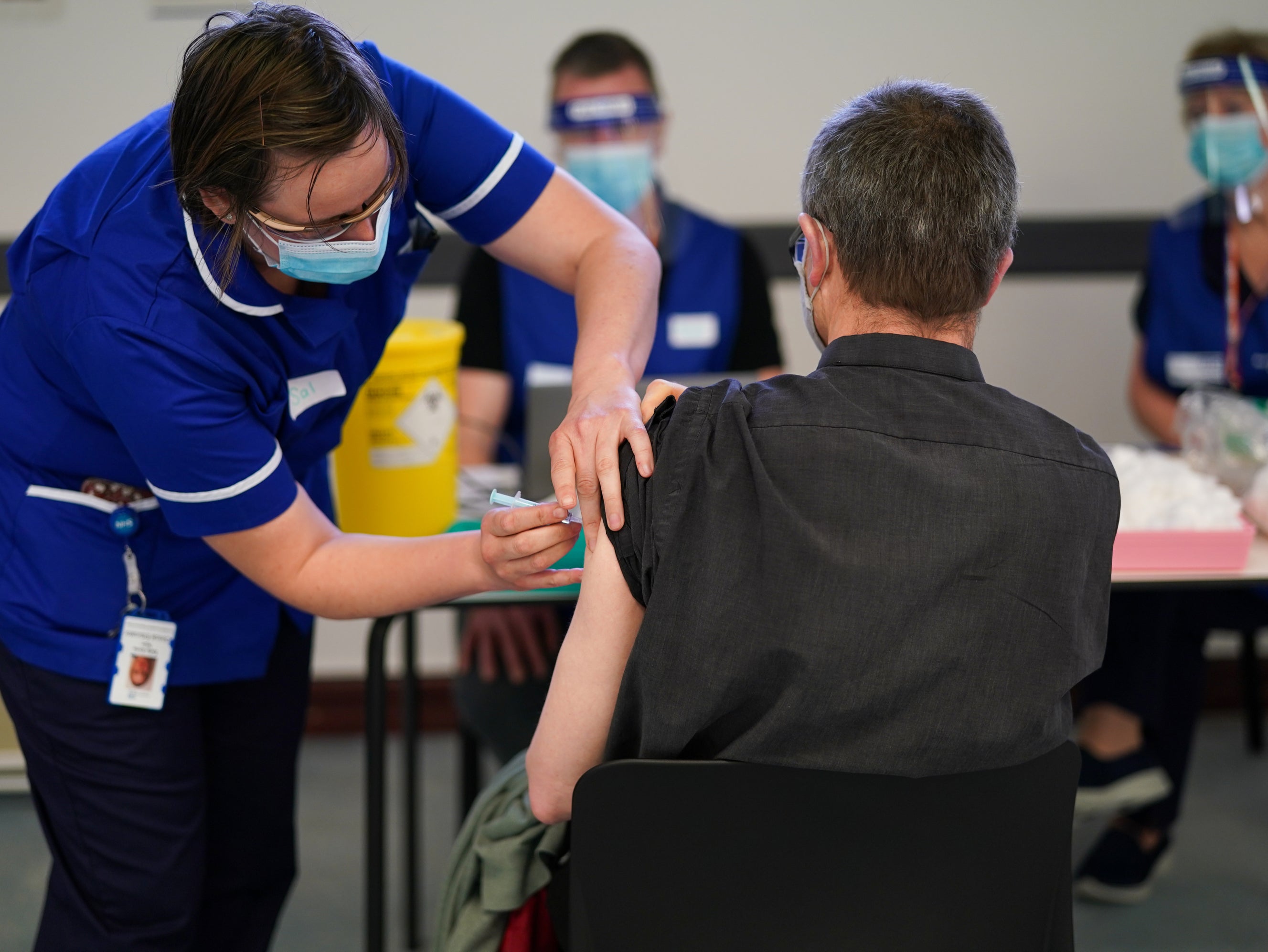
Your support makes all the difference.People who participated in trials for Covid vaccines that have not yet been approved are to be offered two additional doses of the Pfizer jab to allow them to travel internationally, the government has announced.
The policy applies to more than 21,000 individuals and will provide them with the vaccine status needed to visit countries that do not recognise trial vaccinations.
The additional doses will initially be offered to the 15,000 people who trialled the Novavax jab, which has been found to be safe and effective but has yet to be authorised by the UK’s medicines regulator, before being extended to people who have received other vaccines still under review.
These individuals have found themselves in limbo throughout the past year – unable to receive an approved jab during the UK rollout and, as a result, restricted from visiting countries that only grant entry to those who have been immunised with globally recognised vaccines.
But under new guidance provided to the government by the Joint Committee on Vaccination and Immunisation, these people will now be able to receive two doses of the Pfizer vaccine – separated by the usual eight-week gap.
The NHS Covid app will also be altered to recognise the extra two doses and permit international travel.
“The measures we have taken will allow UK Covid-19 vaccine-trial participants to travel freely overseas once they have had the additional vaccinations,” said Professor Jonathan Van-Tam, England’s deputy chief medical officer.
“Those volunteers now have the flexibility to make a decision for themselves, so they can, for example, visit loved ones abroad.”
Professor Paul Heath, principle investigator of the Novavax clinical trial, said: “For too long the participants have been disadvantaged in terms of international travel because this vaccine is not yet approved for deployment.”
The UK recognises those who participated in Covid vaccine clinical trials as fully vaccinated, even if the jab has not been approved; however, the majority of other countries do not.
Through the EU and the World Health Organisation, the government is pushing other nations to change their policy and make it easier for clinical trialists across the world to travel.
“If more countries around the world had reciprocated by allowing UK volunteers to enjoy fully vaccinated status for overseas travel, these measures would not have been necessary,” said Prof Van-Tam.
Trials have been conducted globally on receiving a third vaccine dose, but not a fourth. However, senior health officials do not have any safety concerns, and one-to-one clinical counselling will be provided for vaccine trial volunteers to address any questions they have about receiving additional doses.
There is evidence that mixing three doses of different vaccines is safe, as set out in the Cov-Boost vaccine trial.
If people from the Novavax study are eligible for a Covid booster jab, the first of the two doses that are needed for travel will suffice, health officials said.
Scotland, Wales and Northern Ireland health teams are expected to follow suit for vaccine trial participants in their regions.
In January, Novavax posted positive results for its UK trial, but it has yet to file for approval from the Medicines and Healthcare products Regulatory Agency (MHRA), saying it intends to do so in the next “couple of months”.
The UK has ordered 60 million doses of the vaccine, due to be made in northeast England.
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments