Men could get a new, non-hormonal contraceptive, study shows
Research can help expand men’s birth control options, currently limited to condoms and vasectomy
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.Men could get a new non-hormonal contraceptive that a yet-to-be-peer-reviewed study has said is “99 per cent effective in preventing pregnancy” in mice without obvious side effects.
The study, which will be presented at a meeting of the American Chemical Society (ACS) on Thursday, could potentially expand men’s birth control methods which currently include only two effective options – male condoms and vasectomy.
“Scientists have been trying for decades to develop an effective male oral contraceptive, but there are still no approved pills on the market,” Abdullah Al Noman, a graduate student at the University of Minnesota, said in a statement.
Most male contraceptives currently undergoing clinical trials target the male sex hormone testosterone, scientists said, adding that this could lead to side effects such as weight gain, depression and increased low-density lipoprotein (LDL) “bad cholesterol” levels.
“The female pill has been available for 60 years now, and the male pill has lagged behind,” Gunda Georg, Head of the Department of Medicinal Chemistry at the University of Minnesota, said.
“We wanted to develop a non-hormonal male contraceptive to avoid these side effects,” Mr Al Noman, who works at Dr Georg’s lab and will present the findings at the ACS meeting on Thursday, said.
The experimental contraceptive targets a protein called the retinoic acid receptor alpha (RAR-α), that binds retinoic acid, a form of vitamin A.
Retinoic acid, scientists said, plays important roles in cell growth, differentiation – including sperm formation – and embryonic development.
While earlier research led to an oral compound inhibiting all three members of the RAR family (RAR-α, -β and -γ), causing reversible sterility in male mice, the new study wanted to find a drug specific for RAR-α with less likelihood of side effects.
Scientists closely examined the structures of RAR-α, -β and -γ proteins bound to retinoic acid and identified structural differences in the ways the three receptors bind to it.
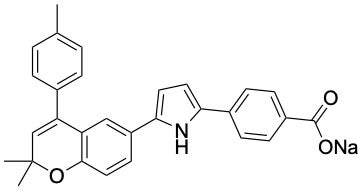
Based on this analysis, they designed and synthesised about 100 compounds and evaluated their ability to selectively bind to RAR-α in cells.
The researchers found “YCT529”, a compound that inhibited RAR-α almost 500 times more potently than it did RAR-β and -γ.
When they orally dosed male mice with the compound for four weeks, scientists claimed it dramatically reduced sperm counts and was “99 per cent effective in preventing pregnancy”.
They also monitored the weight, appetite and overall activity of the mice and did not find any observable side effects.
About a month after the mice were taken off the drug, they could sire pups once more, researchers said.
“Because it can be difficult to predict if a compound that looks good in animal studies will also pan out in human trials, we’re currently exploring other compounds, as well,” Dr Georg said.
The researchers, who received funding from the National Institutes of Health and the Male Contraceptive Initiative and are working with a company called YourChoice Therapeutics, are expecting to start human trials by the third or fourth quarter of 2022.
“There is no guarantee that it will work... but I would really be surprised if we didn’t see an effect in humans as well,” Dr Georg told AFP.
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments