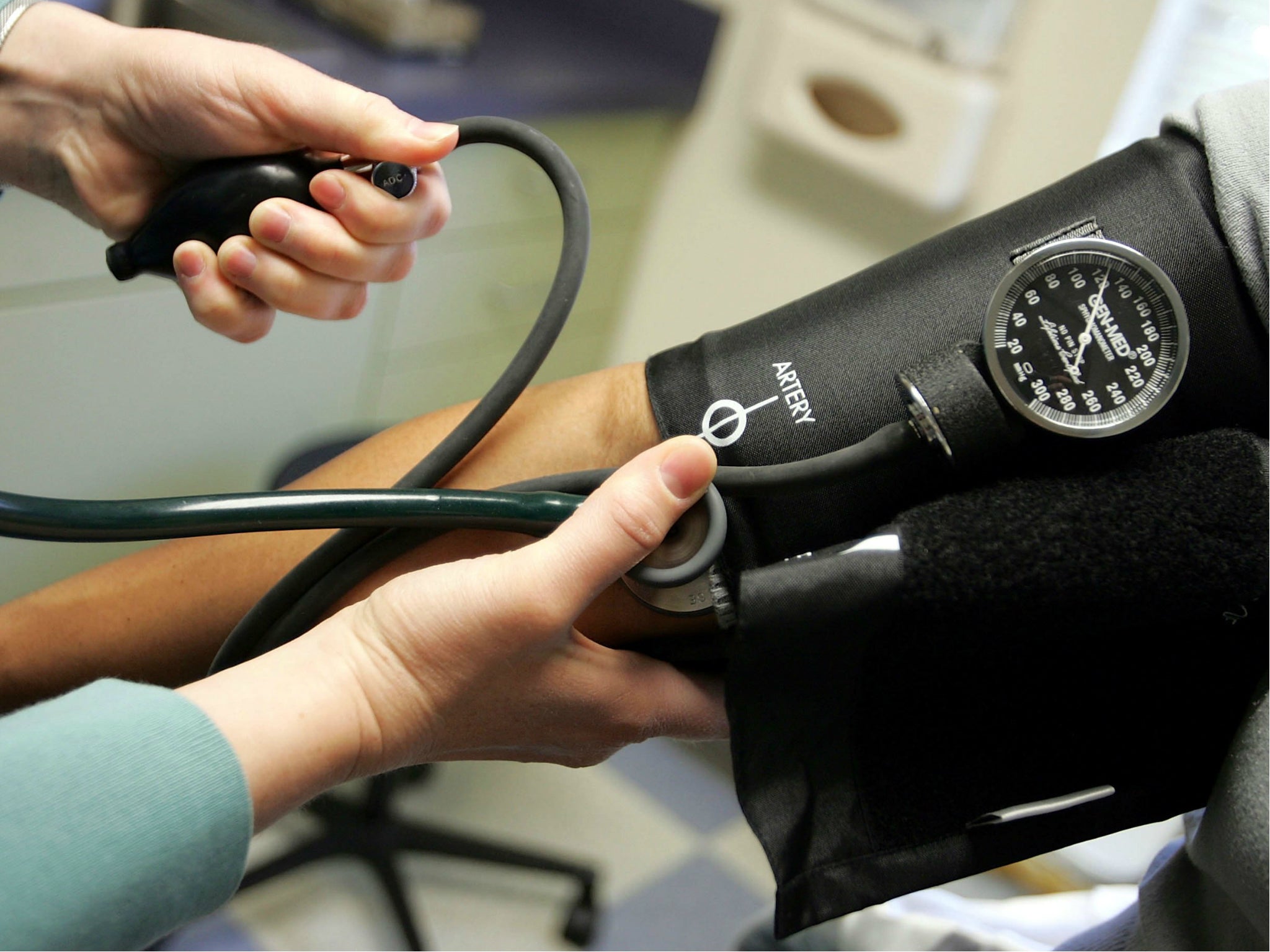
Blood pressure medicine recall expands amid concerns drug might cause cancer
This is just one of several blood pressure medications that have been recalled.
Your support helps us to tell the story
This election is still a dead heat, according to most polls. In a fight with such wafer-thin margins, we need reporters on the ground talking to the people Trump and Harris are courting. Your support allows us to keep sending journalists to the story.
The Independent is trusted by 27 million Americans from across the entire political spectrum every month. Unlike many other quality news outlets, we choose not to lock you out of our reporting and analysis with paywalls. But quality journalism must still be paid for.
Help us keep bring these critical stories to light. Your support makes all the difference.
Another blood pressure medicine is being voluntarily recalled after tests showed it is tainted with a potential cancer-causing chemical.
The US Food and Drug Administration announced the recall for losartan potassium hydrochlorothiazide, made by the company Sandoz.
The active ingredient in the drug contains N-Nitrosodiethylamine (NDEA), a chemical “considered to be reasonably anticipated to be a human carcinogen."
It involves the 100 milligram/25 milligram tablets, but not other versions of the drug, sometimes sold under the brand names Hyzaar. The batch involves the lot number JB8912.
It’s important to note that most blood pressure medication has not been recalled. However, the FDA listed certain lots of Valsartan and Amlodopine as potentially contaminated and recalled as well. The Valsartan-containing pills have been under a recall since July. The drugs were tainted with NDEA or NDMA, N-nitrosodimethylamine, an impurity that is also considered a possible carcinogen. The FDA is currently testing all other related blood pressure medicines for impurities.
The FDA keeps a list of recalled blood pressure drugs non-recalled blood pressure drugs for consumers.
The risk of getting cancer from taking one of these medications is thought to be extremely low. The FDA asks patients to consult their doctors and replacing any medicine that has been recalled.
According to PubChem, NDEA is used in industry materials as an additive to gasoline and lubricant, as an antioxidant, and a stabiliser in plastics. When heated highly enough, it can emit toxic fumes.
NDMA is used to make liquid rocket fuel and is a byproduct of manufacturing some pesticides and processing fish.
Subscribe to Independent Premium to bookmark this article
Want to bookmark your favourite articles and stories to read or reference later? Start your Independent Premium subscription today.
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments