Lab on brink of IVF history - by making groundbreaking technique safe
Wellcome Trust Centre for Mitochondrial Research on brink of IVF history – by making groundbreaking technique safe

There is an air of quiet excitement when you enter the Wellcome Trust Centre for Mitochondrial Research in the medical school of Newcastle University. It’s a feeling that the time has finally come for its scientists on their long journey to help couples give birth to babies free of mitochondrial disease.
Next week’s vote in the House of Commons will effectively determine whether the centre will be allowed to apply for a licence permitting it to carry out mitochondrial transfer, commonly known as the “three-parent” baby technique for creating IVF embryos.
Mitochondria, the microscopic “power packs” of cells, have their own DNA which is passed down the maternal line. When these mitochondrial genes are defective they result in a range of disorders. About one in every 6,500 babies inherits severe forms of mitochondrial disease.
The centre’s director, Professor Doug Turnbull, exudes the energy and enthusiasm of a sports coach in his tracksuit top as he gives me a whistle-stop sprint around his labs. He wants me to see everything, answer any questions, but there is one thing he cannot talk about.
What is it that you have done, I asked him, since you last published research on this subject in 2010 to show that the technique will be safe? It takes at least 10 seconds of almost embarrassing silence before he breaks into a nervous laugh.
“I can say they are very encouraging,” he replied, finally. “I wouldn’t be saying that if … [another pause]. I’ve got to be careful about what I say because it will go to some high-impact journal and they will not want me to say anything,” he added.
What he was referring to is a five-year scientific study that he and his university colleague Professor Mary Herbert have just completed, but not yet submitted to a peer-reviewed science journal. This research could be the crucial evidence needed to convince the regulatory body, the Human Fertilisation and Embryology Authority (HFEA), that mitochondrial transfer is safe for its first IVF licence – once MPs vote on its legality.
The Newcastle technique is called pro-nuclear transfer and involves moving the nucleus of a fertilised egg into a donated fertilised egg with its own nucleus removed. In this way, the nuclear chromosomes of the mother and father are added to the healthy mitochondrial genes of the woman who donated the egg.
While some will argue about the ethics of creating fertilised eggs solely to be used as a treatment for IVF embryos, the science is focused on safety and risk. This is not just directed at the mothers and the first generation of children born from the technique, but to their grandchildren and even their grandchildren’s children. This is because mitochondrial transfer will be the first medical procedure that allows the modification of the human germline – the deliberate alteration of the genetic makeup of all subsequent generations within an affected family.
This cross-generational aspect of the technique is not something that is easily tested in a laboratory, and other scientists abroad have urged a more cautious approach – involving more animal experiments, particularly on monkeys.
But this is not under consideration in Britain, where the emphasis is on working with human eggs and embryos in a laboratory. “What we focused on predominately is whether or not we’ll get a successful pregnancy because at the end of the day that’s what’s going to be critical,” Professor Turnbull said.
“This is the only option available for some women and if it prevents disease in their offspring and, potentially, in their offspring to follow, I think it is a reasonable, sensible way forward,” he said.
A short taxi ride away on the other side of Newcastle city centre I met with Mary Herbert, professor of reproductive biology at the university’s Institute of Genetic Medicine. She showed me the incubators where the human eggs and embryos will be manipulated before being transplanted into the first patients.
I raised the issue of this procedure changing the human germline – of it being essentially a form of genetic modification – and asked what she thinks of the “three-parent baby” shorthand used in the press.
“What we do here is create new combinations of mitochondrial and nuclear genomes. That happens every time an egg is fertilised. It happens in nature and I think we would not be here without it,” Professor Herbert said. “It’s certainly not genetic modification. If this is genetic modification then we are all genetically modified organisms.”
And the three-parent baby description?
“It’s a media term and it comes down to the definition of a parent. The people who come for IVF treatments see the scans and the heartbeats of the baby, these are the people who are the parents,” she replied.
I also ask about her unpublished study where she has used healthy human eggs to create the manipulated embryos. “We’re very, very encouraged by it. I think it’s really within reach to offer this as clinical treatment to prevent the transmission of mitochondrial disease,” she replied, wary that she too must not give too much away before publication in a peer-reviewed journal.
If Parliament next Tuesday approves the government proposal to permit mitochondrial transfer, the HFEA will be legally allowed to consider issuing an IVF licence to Newcastle in October, when the new regulations come into effect.
An HFEA scientific panel has already seen the preliminary results of the Turnbull-Herbert study and appears to have been impressed by their findings, albeit with one unexplained caveat.
“This work has identified some subtle differences in embryo development that are being investigated, but nothing has been found so far which raises concerns about safety,” the panel reported last year.
However, the experts have changed their mind about what needs to be done to assess safety. Back in 2011, the panel said it would be “critical” for experiments on monkeys to show that the offspring derived from mitochondrial transfer are normal.
However, in 2013 the panel had formed a different opinion, saying that it was no longer “critical or mandatory” for animal experiments on non-human primates – monkeys. Experiments on human eggs and embryos would suffice, it said.
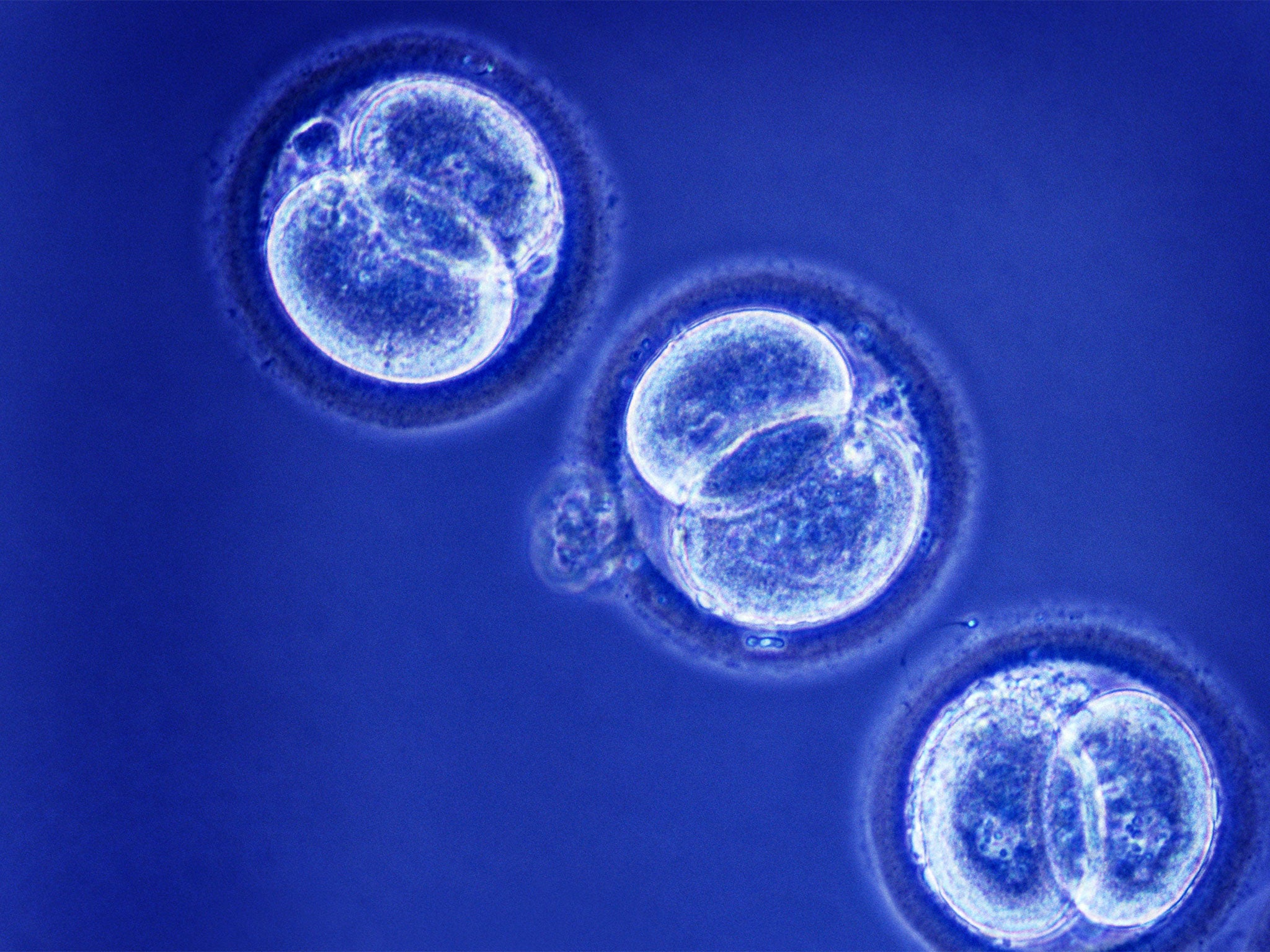
This goes against the scientific advice given to the US Food and Drug Administration, which is also considering mitochondrial transfer. Its advisers believe that at least two further years of research on animals, including monkeys, will be needed before the technique can be approved for human IVF in America.
Other scientists also want to see further work on monkeys, and in particular their offspring.
“It is essential to analyse offspring to determine that no abnormalities appear at least during early life,” said Professor Justin St John of Monash University in Australia.
Researchers in the US, led by Shoukhrat Mitalipov of Oregon Health and Science University, are working on a different technique for carrying out mitochondrial transfer – known as maternal spindle transfer – and have already demonstrated in macaque monkeys that the offspring were healthy.
However, when Dr Mitalipov tried to do the same with pro-nuclear transfer – the technique used in Newcastle – it failed to work in macaques.
This was why the HFEA panel decided to drop its insistence on the need for primate experiments on pro-nuclear transfer, citing differences between human and macaque reproduction.
Professor Herbert in Newcastle believes this was the correct decision. “I think when it comes to the small print there are differences between humans and primates, I would prefer to continue to work in humans,” she said.
The Newcastle team has instead created human IVF embryos using pro-nuclear transfer for research purposes and developed them to the blastocyst stage, when they form a hollow ball of cells at between five and 10 days after fertilisation.
By comparing these “three-parent” blastocysts with normal blastocysts, Professor Herbert said it is possible to judge whether the technique of mitochondrial transfer is likely to result in a successful transplantation in the womb – and a healthy pregnancy.
“We’re in quite a strong position to understand what are the chances of implantation from the work we’re doing now. We’ve made a lot of progress over the past two years although I can’t talk about it because it hasn’t been published yet,” she said.
And if the vote on Tuesday goes against mitochondrial transfer?
“It will be a big setback for sure, very disappointing for the families who need these treatments,” she replied.
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments
Bookmark popover
Removed from bookmarks