IVF embryo gene-editing Q&A: What is being proposed? Will this lead to 'designer babies'?
Scientists want to use the new and incredibly efficient Crispr/Cas9 technique
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.What is being proposed?
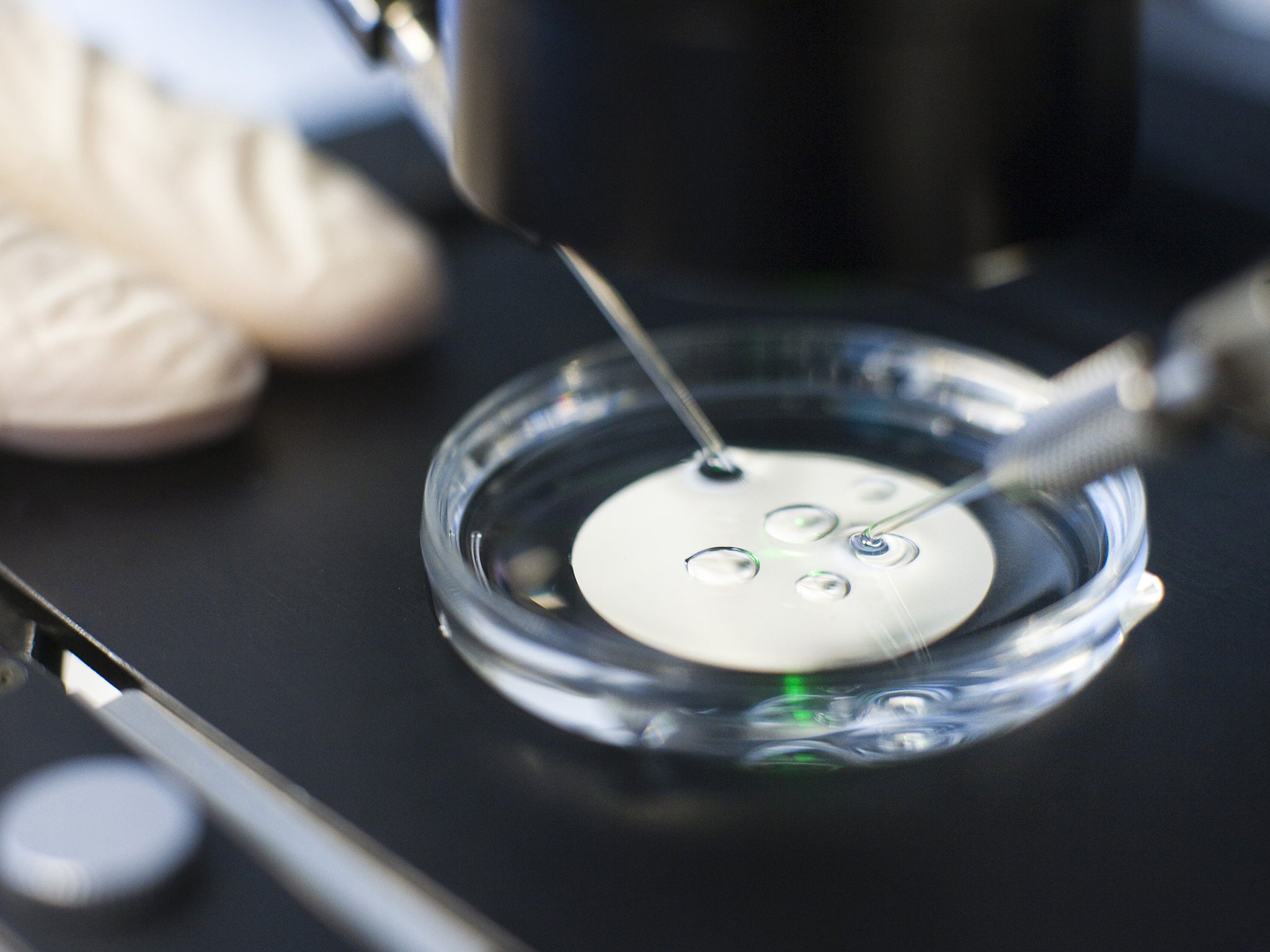
Scientists want to use a new and incredibly efficient method of “gene editing”, known as Crispr/Cas9, to alter the genes of IVF embryos. They specifically want to change or delete certain genes in the outer cells or “trophectoderm” of the early human embryo. These are the cells that go on to form the placenta which attaches to the uterus when the embryo becomes implanted in the womb during pregnancy.
Why are they doing this?
The researchers want to understand the genetic mechanism that underpins the development of the placenta. By doing this, they hope to be able to find out what goes wrong in women who suffer repeated miscarriages, which may be the result of irregularities in the way the placenta forms during the crucial early stages if embryonic development. They also want to use it for generating embryonic stem cells.
Will this lead to GM babies?
No. This licence application is for research purposes only. It will be carried out on spare IVF embryos donated by couples undergoing fertility treatment. The embryos are less than 14 days old and will be destroyed once the project is finished – it will be illegal to transfer them into the womb.

Can’t this be done in some other way?
A lot of work in this area is done on mice, but scientists know that the early development of the trophectoderm in mice embryos is significantly different to the development of human embryos. Because of these differences, the scientists would like to work directly on human IVF embryos by, for instance, deleting certain genes using Crispr/Cas9 to see how this affects the development of the tissue that gives rise to the placenta.
Why is this controversial?
Crispr/Cas9 is so powerful some see it as a neat way of safely engineering the human genome. Doing it in eggs, sperm or human embryos used in fertility treatment – which is currently illegal – will alter the “germline” DNA passed on to future generations. If ever it were to be allowed, it raises the prospect of ridding inherited disorders or conferring disease resistance to future generations, but could also usher in an era of genetically-enhanced “designer babies” engineered with desired traits, such as intelligence or athletic ability.
Subscribe to Independent Premium to bookmark this article
Want to bookmark your favourite articles and stories to read or reference later? Start your Independent Premium subscription today.
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments