The Independent's journalism is supported by our readers. When you purchase through links on our site, we may earn commission.
Scientists genetically engineer algae that can kill cancer cells while leaving the rest of the body unharmed
The new medical technology could one day help patients with types of cancer that are currently untreatable
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.A team of Australian and German scientists have managed to genetically engineer algae that can kill cancer cells while leaving normal cells unharmed.
Existing cancer treatments, including many chemotherapy remedies, kill cancer cells but also harm healthy cells, causing unpleasant side effects for patients.
So, as the researchers put in in their study, which was published this week in the high-profile Nature Communications journal, "the ability to selectively kill cancerous cell populations while leaving healthy cells unaffected is a key goal in anticancer theraputics."
Scientists have had some success in this area by using silica-based nanoparticles, but the production process is expensive, and involves the use of toxic chemicals.
Now, by using lower-cost and natural silica-based algae nanoparticles, the researchers have been able to deliver anti-cancer drugs to cancer cells without harming the body's healthy cells.
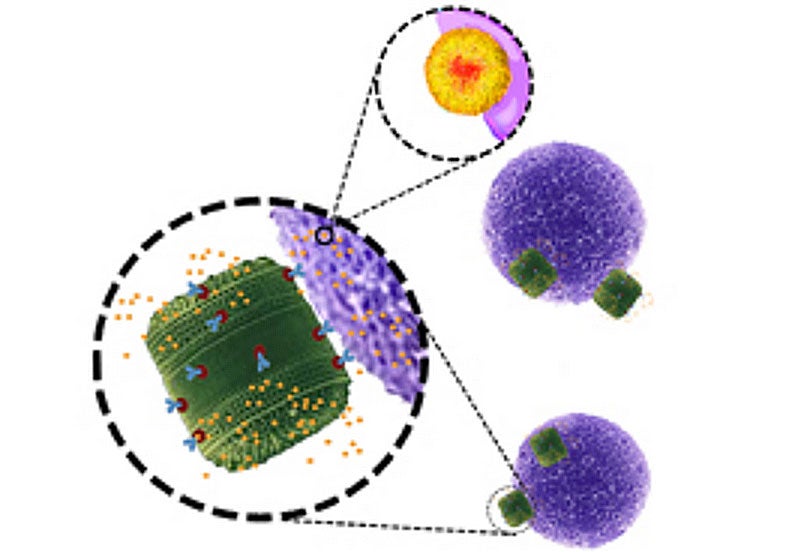
The scientists produced these particles by genetically engineering diatom algae, tiny single-celled algae organisms that are unique due to their cell walls, which encase the inner parts of the cell with silica.
They altered the algae so that the silica coatings of the cells were covered with a protein that binds to antibodies.
These antibodies, which make up a large part of the human immune system, then bind to the cancer cells that are attacking the body.
By 'hiding' the anti-cancer drugs within the algae, the scientists ensured that the drugs could then be delivered directly to cancer cells without coming into contact with healthy cells.
As they put it in the study, the algae cells act as 'backpacks', that bind to antibodies, which in turn bind to cancer cells, killing them but leaving normal cells without a scratch.
Unlike the costly and toxic nanoparticles that have previously been produced, algae only needs water and light to grow - making it a lower-cost and much less harmful drug delivery system.
It's still very early days for this new technology, but it holds a lot of potential - particularly for sufferers of certain brain tumours, which are currently untreatable.
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments