Dmitri Mendeleev: Five facts you possibly didn't know about the periodic table
The table got four new elements in January

Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.Dmitri Mendeleev, the Russian chemist who published what is regarded as the first widely recognised periodic table, has been celebrated with his own Google doodle on what would have been his 182nd birthday.
The inventor formulated the periodic law – which states that elements can be arranged by their mass and be organised into groups that share similar chemical and physical properties.
Mendeleev’s “eureka moment” came when he realised that there was a system underpinning the properties of all the elements. As a result, not only was he able to organise the known elements into a table, but – crucially – he realised that some elements existed that had yet to be discovered.
He published his Periodic Table – in all its incomplete beauty - in 1869.
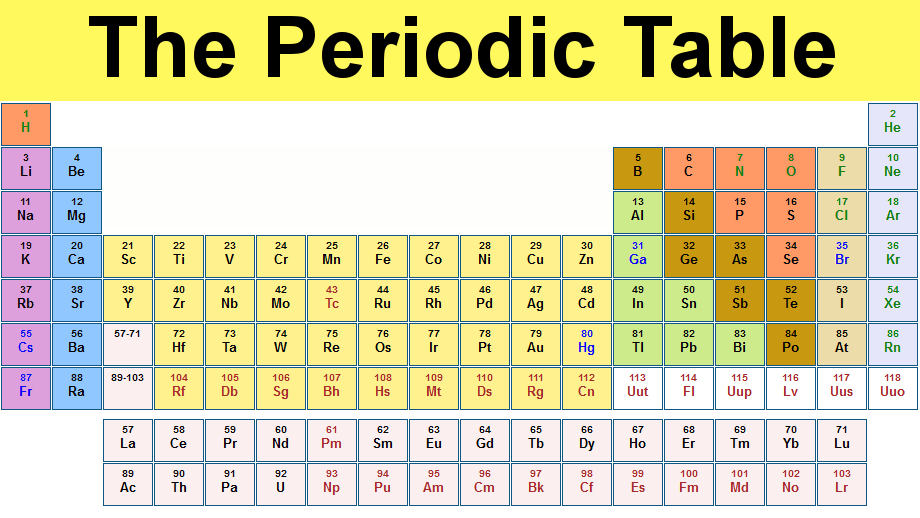
The table consists of 118 elements, which are organised by their atomic numbers.
An atomic number counts the protons within the nucleus of an atom which - along with neutrons - make up the majority of the weight of an atom.
It is divided into periods (the rows) and groups (the columns). Six of the groups have their own name – perhaps the most well-known of these being the noble gases (group 18).
Here are five facts you might not know about it (despite all those hours spent in science class) and Mendeleev himself:
The periodic table gained four new elements in one day
On 4 January, it was confirmed that elements 113, 115, 117 and 118 would be added to the table’s seventh row to make it complete after they were verified by the International Union of Pure and Applied Chemistry on 30 December. They have been worked on since at least 2004, when studies began showing the discovery and priority of element 113. But they have all now satisfied the strict tests to be admitted to the periodic table. They are yet to receive their final names or symbols.
Mendeleev wasn’t the only one working on a Periodic Table.
The German physician Lothar Meyer published – in 1964 – an early prototype version of the Periodic Table in which he grouped together elements with similar chemical and physical properties. By 1868 he had identified 53 elements, but this version was not published until 1895 – nearly three decades after Mendeleev’s version. Despite being a bit late to the party, Meyer is widely recognised as having made an important contribution to the development of the table.t.
A lot of them are man-made
Ninety of the elements are found naturally. Of the two dozen or so that aren't, the first to be made artificially was Technetium. This was discovered in 1937 by Carlo Perrier and Emilio Segre and was named after the Greek for artificial - technetos. It is one of the elements used as the radioactive part of the tracers put into the human body during medical tests.
Mendeleev hasn’t had the final say
Ever the tinkerers, scientists have been trying to improve upon Mendeleev’s version of the table. Perhaps one of the most interesting is the one conceived of by the German philologist Theodor Benfey, who created a two dimensional spiral in 1964. Benfey’s version is designed to illustrate the disproportionate distance between elements at opposite ends of Mendeleev’s version (consider the standardised map of the London Underground network, which while the best version to understand London’s sprawling Tube network, bears little resemble to the physical distances between the various stations).Paul Giguere’s three dimensional periodic table is more complex yet. But neither of them – nor the multitudinous other versions - have managed to dislodge Mendeleev’s from school textbooks.
Terry Pratchett fans have got involved
After the death of the author Terry Pratchett, tens of thousands of fans signed a petition calling for a an element in the periodic table to be named in honour of him. Element 117 has been given a temporary symbol of Uus, but a petition instigated by Dr Kat Day, a chemist, blogger and self-proclaimed “huge” Pratchett fan, wants 117 to be named octarine [Oc] as a tribute to Pratchett, who passed away aged 66 from Alzheimer's disease in March 2015. Octarine is the colour of magic in the late author's Discworld series.
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments