UK researchers to investigate Ivermectin as possible Covid-19 treatment
The anti-parasitic drug is already used controversially in some countries to treat people, despite limited evidence of its effectiveness
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
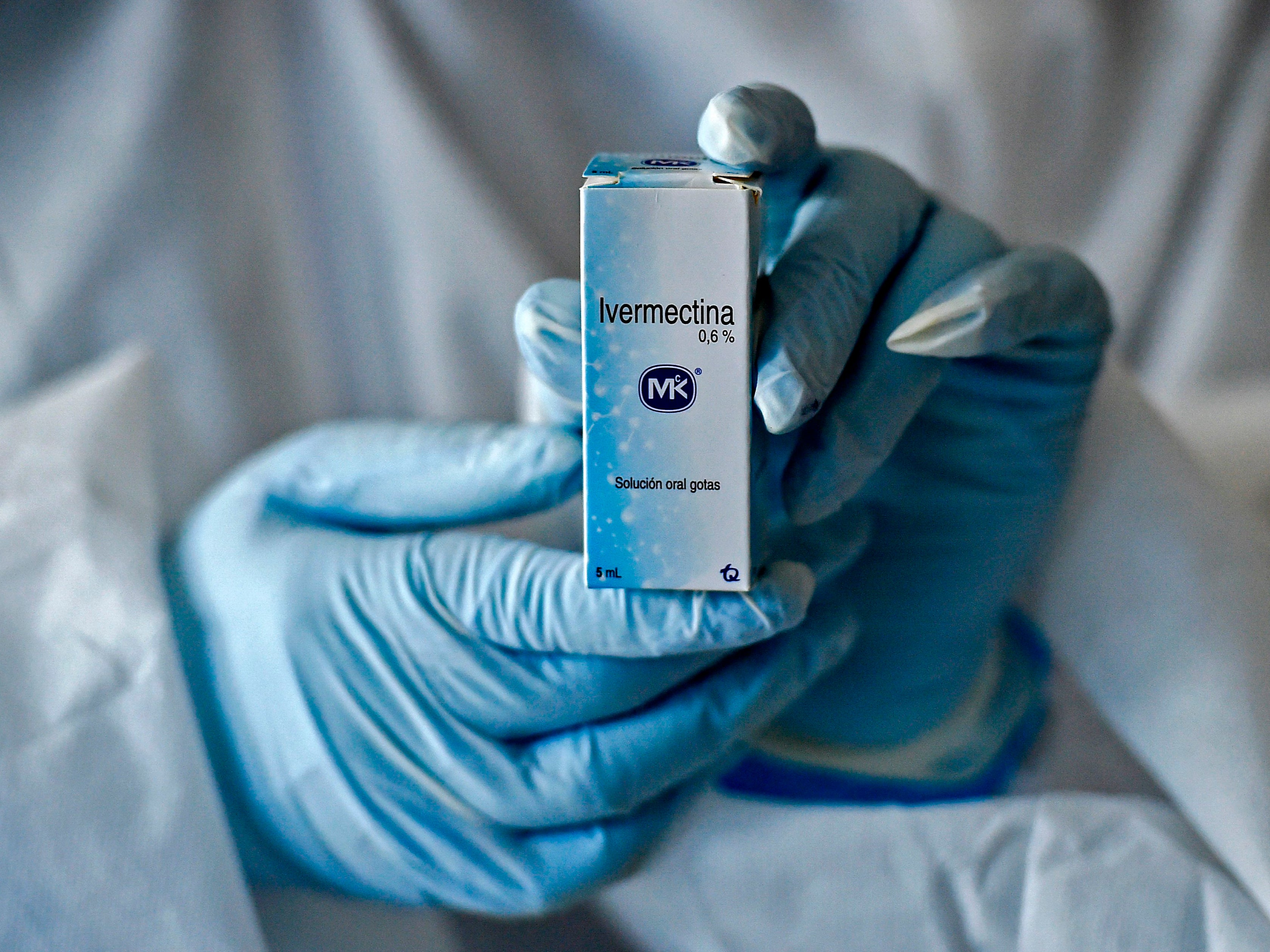
Your support makes all the difference.Ivermectin, a widely used anti-parasitic drug, is to be investigated as a possible treatment for Covid-19, researchers have announced.
The drug, used across the world to treat parasitic infections, will be studied as part of Oxford University’s Principle trial - which is dedicated to finding at-home medicine for speeding up recovery from or preventing hospitalisation with Covid-19.
Ivermectin has been controversially touted as a potential treatment for Covid since the earliest stages of the pandemic.
Although the drug is not an antiviral, laboratory-based studies have found that it can block replication of Sars-CoV-2 - the virus that causes Covid-19 - but at much higher, and unsafe, concentrations than those used in currently authorised ivermectin treatments.
Results from clinical studies were varied, with some studies showing no effect and others reporting that ivermectin has a potential benefit, in terms of reducing viral load and the duration of symptoms in some patients with mild Covid.
However, the lack of evidence from large-scale, randomised controlled trials has made it impossible to say with confidence that the drug is effective in treating Covid - though some countries, such as Peru, Bolivia and Colombia, have decided to nonetheless push ahead with administering ivermectin to patients.
The US Food and Drug Administration has previously warned against using ivermectin to treat or prevent Covid-19, while a European Medicines Agency review in March concluded that “the available data do not support its use for Covid-19 outside well-designed clinical trials”.
Oxford’s professor Chris Butler, the joint chief investigator of the Principle trial, said his team hopes “to generate robust evidence to determine how effective the treatment is against Covid-19, and whether there are benefits or harms associated with its use.”
He said the drug is readily available on a global level and is well known to have a “good safety profile”.
Participants enrolled in the study will be randomly assigned a three-day course of ivermectin treatment. They will be followed-up for 28 days and will be compared with trialists who receive the usual standard of NHS care only.
Dr Stephen Griffin, a virologist at the University of Leeds, said the drug’s inclusion in the Principle trial “should provide a final answer to the questions over whether this drug might be repurposed as an antiviral targeting Sars-CoV-2”.
“Much like hydroxychloroquine before, there has been a considerable amount of off-label use of this drug, based primarily upon in vitro cell culture data,” he said.
“However, antiviral effects have only been demonstrated in such systems at concentrations much higher than those corresponding to routine anti-parasitic treatment.”
Ivermectin is the seventh treatment to be investigated by the Principle trial, and is being evaluated alongside the influenza antiviral favipiravir.
In April, researchers from the study found that budesonide, a medicine used for asthma, can shorten the recovery time of Covid-19 sufferers who do not need hospital treatment by an average of three days.
The readily available drug, administered via a cheap inhaler twice a day for up to 14 days, is being prescribed by GPs on a “case-by-case basis”, allowing doctors to treat Covid-19 patients at home.
Ivermectin’s admission to the Principle trial will be closely monitored by the government’s taskforce dedicated to searching and developing a new generation of drugs capable of treating people with coronavirus in their own homes.
The taskforce, modelled on the team responsible for the UK’s vaccine programme, is aiming to identify and support research into promising antiviral treatments that can reduce transmission and speed up recovery from Covid-19.
It’s hoped two effective treatments – offered in tablet form – will be made available to the public as early as autumn, providing Britain with “another layer of defence” alongside the vaccines in combating possible future waves and emerging variants, said Sir Patrick Vallance in April.
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments