Scientists discover completely new form of carbon after decades of searching
'We now have the recipe for how to make these structures'
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.A new form of carbon has been created, opening up a whole new world of potential technological applications.
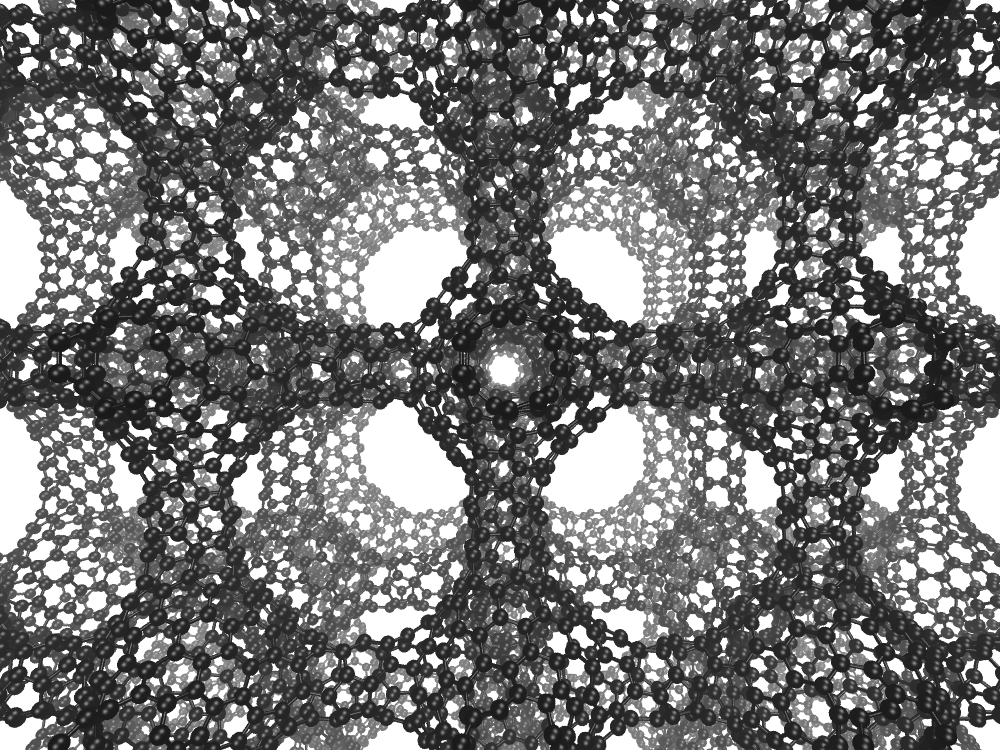
Carbon can already be moulded into precious diamonds, the graphite in pencils and graphene – the strongest material on Earth – and “schwartzites” are the latest addition to this family.
Schwarzites have long been predicted by chemists, who have suggested they would have unique properties that make them useful in batteries and as catalysts.
In a surprising turn of events, after decades of searching these theoretical materials were accidentally created by researchers working in South Korea and Japan.
The scientists were investigating zeolite-templated carbons (ZTCs) – crystalline forms of silicon dioxide with carbon structures built inside them – and checking for any interesting properties.
In doing so, they unintentionally made schwarzites, but their creation was ignored until University of California, Berkeley chemists noticed their unusual structure.
"We now have the recipe for how to make these structures, which is important because, if we can make them, we can explore their behaviour, which we are working hard to do now," explained Professor Berend Smit, a chemical and biomolecular engineer who led the research.
Carbon can be arranged into 2D “crystals”, including sheets of graphene and football-shaped fullerenes, which are defined by how their structures curve when the carbon atoms lock together.
Unlike fullerenes – which are positively curved – and graphene – which is not curved at all – schwartzites are negatively curved.
"The experimental validation of schwarzites thus completes the triumvirate of possible curvatures to graphene; positively curved, flat, and now negatively curved," said Professor Braun.
Zeolites, minerals more commonly used to soften water in laundry detergents, turn out to be the key component in taking this carbon form from theory into reality.
In their paper, published in Proceedings of the National Academy of Sciences, Professor Braun and his team confirmed it is possible create these structures by injecting a vapour containing carbon into zeolites.
Once inside, the carbon assembles into a graphene-like sheet that lines the walls of the zeolite pores. In doing so, the surface stretches and bends negatively.
The zeolite can then be dissolved to produce pure schwartzite.
“The schwarzites synthesised to date have been made by choosing zeolite templates through trial and error,” said Professor Braun.
“We provide very simple instructions you can follow to rationally make schwarzites and we show that, by choosing the right zeolite, you can tune schwarzites to optimise the properties you want."
Optimisation of these structures could prove highly beneficial. In the past, discovering new forms of carbon has yielded exciting technological revolutions and earned two Nobel Prizes.
Though the application of schwartzites remains to be seen, the scientists speculate their electronic, magnetic and optical properties could make them useful in the electronics and fossil fuel industries.
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments