South Africa approves Pfizer vaccine booster amid COVID wave
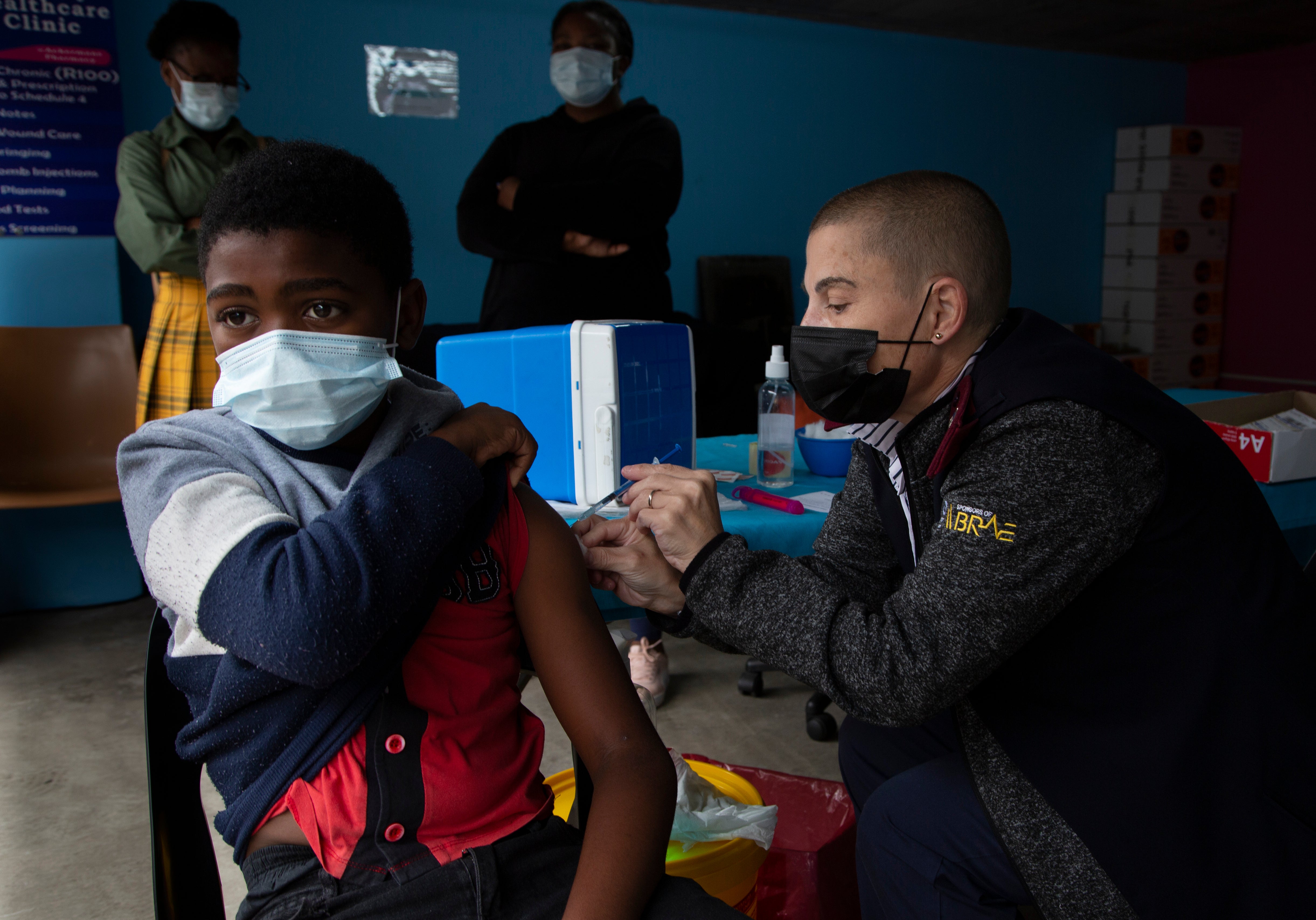
South Africa’s regulatory authority has approved the Pfizer-BioNTech vaccine as a booster shot, opening the way for third doses to be administered to battle the current surge driven by the omicron variant
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.South Africa’s regulatory authority has approved the Pfizer-BioNTech vaccine as a booster shot, opening the way for third doses to be administered to battle the current surge driven by the omicron variant.
South Africa’s new COVID-19 cases continue to rise. In the last 24 hours, South Africa recorded 22,391 new cases, up from about 200 per day in early November. More than 90% of the new cases are omicron, according to genetic sequencing surveys.
The South African Health Products Regulatory Authority approved the Pfizer vaccine as a booster shot for people 18 years and older, six months after they received their second dose.
The regulatory body also approved a third dose for people aged 12 years and older who were “severely immunocompromised,” which may be taken 28 days after their second dose.
The regulatory body encouraged vaccine manufacturers to provide data regarding the use of different vaccines on an individual, known as “mix-and-match.”
The approval came a day after Pfizer announced that a laboratory study showed its two-dose COVID-19 vaccine had reduced efficacy against the omicron variant but said that a booster shot was still effective against omicron.
On Thursday President Cyril Ramaphosa met with the COVID-19 ministerial advisory committee to discuss the rising number of infections and the impact of the spread of the omicron variant.
Ramaphosa and his expert advisers were also expected to discuss the burning issue of whether the government should make vaccines mandatory. Some private companies and universities have already announced they will implement vaccine mandates.
Despite having adequate supplies of more than 19 million vaccine doses, South Africa's vaccination campaign has lagged. Just over 14 million South Africans have been fully vaccinated, representing 36% of the adult population and about 24% of the total population. The relatively slow rate of vaccinations makes the government's goal of inoculating 67% of the population by February look unrealistic.