Monkeypox: New treatment trial launched by Covid researchers
‘It’s crucial that we invest in developing, refining and evaluating treatments for this disease’, says chief trial scientist
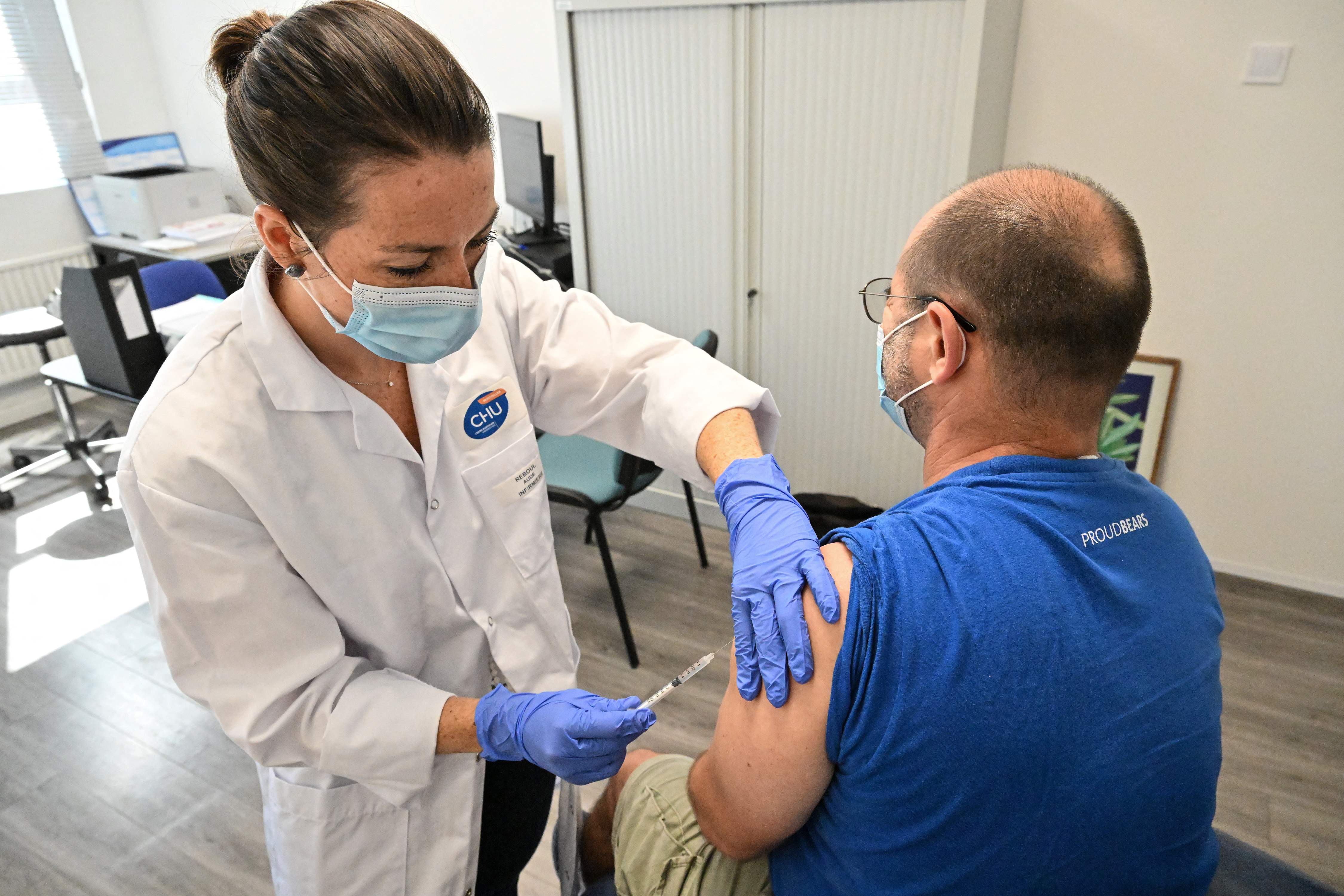
New medication to treat monkeypox is to be trialled by a team that leads on breakthrough Covid treatments.
The drug, which could help speed up recovery for those infected by monkeypox, is being trialled by a team of scientists at Oxford University after it received £3.7m from the National Institute for Health and Care Research (NIHR).
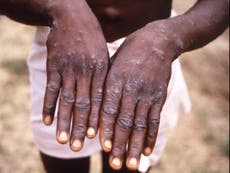
The medication, called tecovirimat, was originally developed as a treatment for smallpox and aims to prevent the virus from leaving infected cells, halting its spread.
The Medicines and Health products Regulatory Agency (MHRA) licensed the drug for use for monkeypox based on the results of studies in animals and evidence indicating it was safe for healthy human volunteers.
It is being used by hospitals for those with severe monkeypox complications but there have yet to be any clinical trials to confirm if it can help people recover from the disease.
Professor Lucy Chappell, chief executive of the NIHR and chief scientific adviser at the Department of Health and Social Care, said: “This study is a very important next step towards looking at treatments for monkeypox outside of hospital.
“It’s crucial that we invest in developing, refining and evaluating treatments for this disease. We have commissioned this research to show the sense of and seriousness with which the health research community is collectively approaching this issue.”
The news comes after UK Health Security Agency announced this week that three pilots have been launched offering patients smaller doses of the monkeypox vaccine amid concerns over shortages.
Harun Tulunay, a training and volunteer coordinator for Positively UK, a charity that supports those living with HIV, had the monkeypox treatment after being diagnosed in June.
Tulunay said: ‘When you are really sick and in pain, you are ready to try anything, but you do want to know if it is going to work or not. I was at the Royal Free London, very much suffering from monkeypox when my doctors offered me tecovirimat. They told me there were no data on how efficient it was, but after days, even weeks, of no improvement in my health, I still wanted to take it. And for the first time, my health improved and I started to feel better.
“I don’t want anyone else to become as severely sick as I was… Knowing whether there is an effective treatment may also help to reduce any anxiety around monkeypox.”
The study, called Platinum, will cover 500 participants and will either give them a 14-day course of tecovirimat twice daily or a placebo treatment.
Participants will take treatments in their own homes and eligible patients will be identified through positive monkeypox tests.
The study is being led by Sir Peter Horby, professor of emerging infections and global health at the University of Oxford, and Sir Martin Landray, professor of Medicine and Epidemiology at Oxford Population Health, who were chief investigators of the Covid-19 Recovery trial.
Prof Sir Peter Horby said monkeypox was a distressing and sometimes dangerous infection and the trial would benefit current and future patients worldwide.
“Although the early data on tecovirimat are promising, only a randomised clinical trial will provide the level of evidence we need to treat patients with confidence. Platinum will provide that evidence,” he said.



Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
0Comments