PrEP: HIV prevention drug trial to double, with 13,000 extra patients to benefit
‘It has been completely unacceptable to see people in need being turned away from clinics’
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.A drug that prevents HIV is to be offered to an extra 13,000 patients, health chiefs have announced.
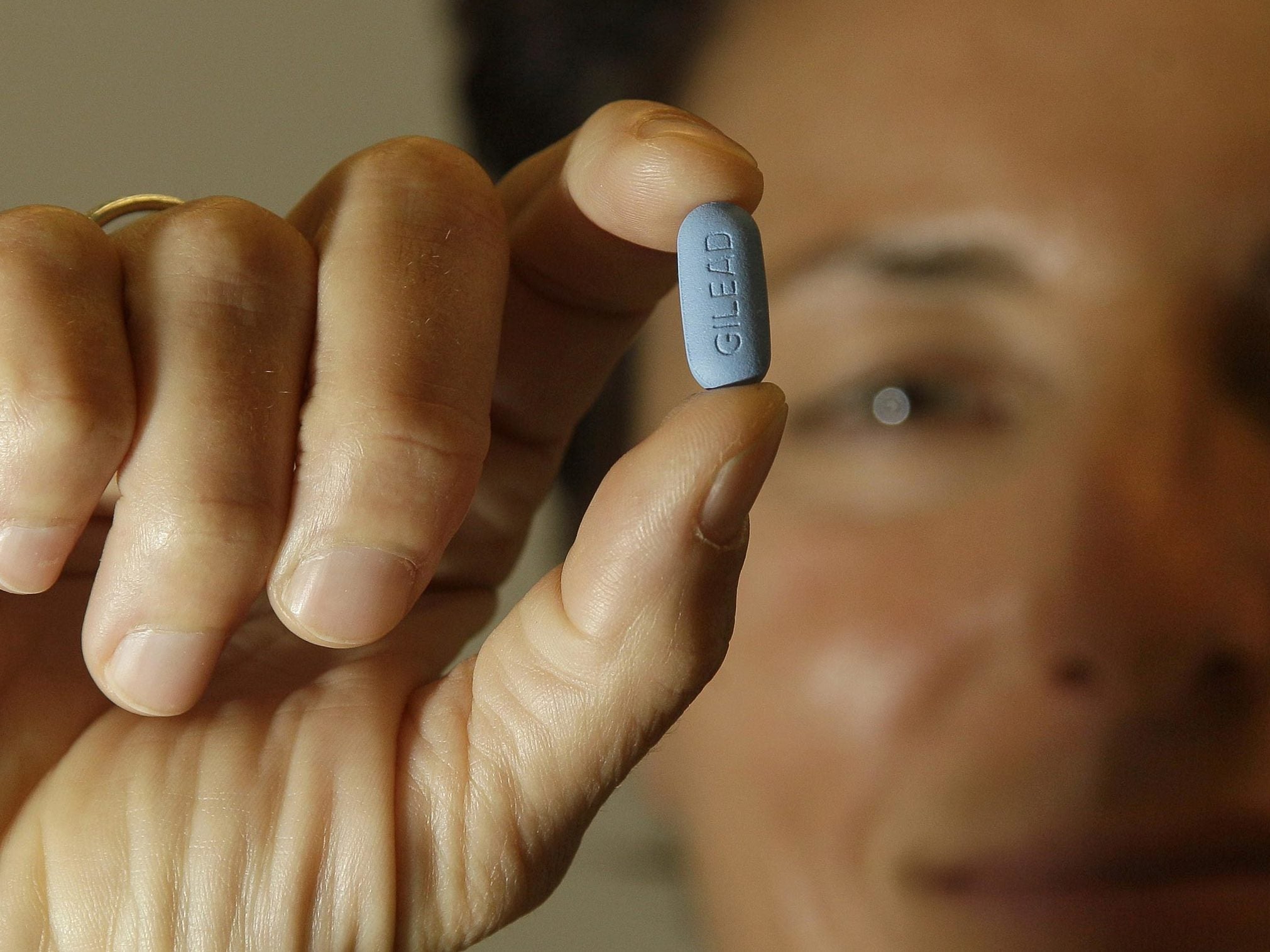
NHS England is providing cash to double the size of a trial of PrEP, which studies show is up to 99 per cent effective in stopping HIV taking hold in the body when transmitted.
The trial already involves 13,000 people but more have wanted to join, and the cap on the number of places available to clinics has meant that some people are being turned away, according to the National Aids Trust.
Some of those people have gone on to contract HIV, the trust believes.
Health bosses say implementation of the trial has been a huge success.
In Scotland, Northern Ireland and Wales, no limitations have been placed on the numbers who can access the drug.
The trial researchers are planning for a full national programme of the drug to be rolled out in partnership with local authorities.
They say expanding the trial until in finishes in 2020, involving 26,000 participants, will help answer questions about the need for PrEP among women.
PrEP (HIV pre-exposure prophylaxis), sold under the brand name Truvada among others, is taken before sex to prevent infection with HIV.
The trial expansion will require approval from an oversight board, due to meet later this month.
Deborah Gold, chief executive of NAT (National Aids Trust), said: “It has been completely unacceptable to see people in need of PrEP being turned away from clinics.
“PrEP is one of the additional tools we have always needed to reverse the spread of HIV, and these extra places will mean that fewer people acquire the virus.”
She said work remained in making sure that everyone who might benefit, especially women and heterosexual men, knew that there was an effective HIV prevention pill.
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments