Moderna becomes third Covid vaccine to be approved for use in UK
First doses expected to reach UK in spring
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
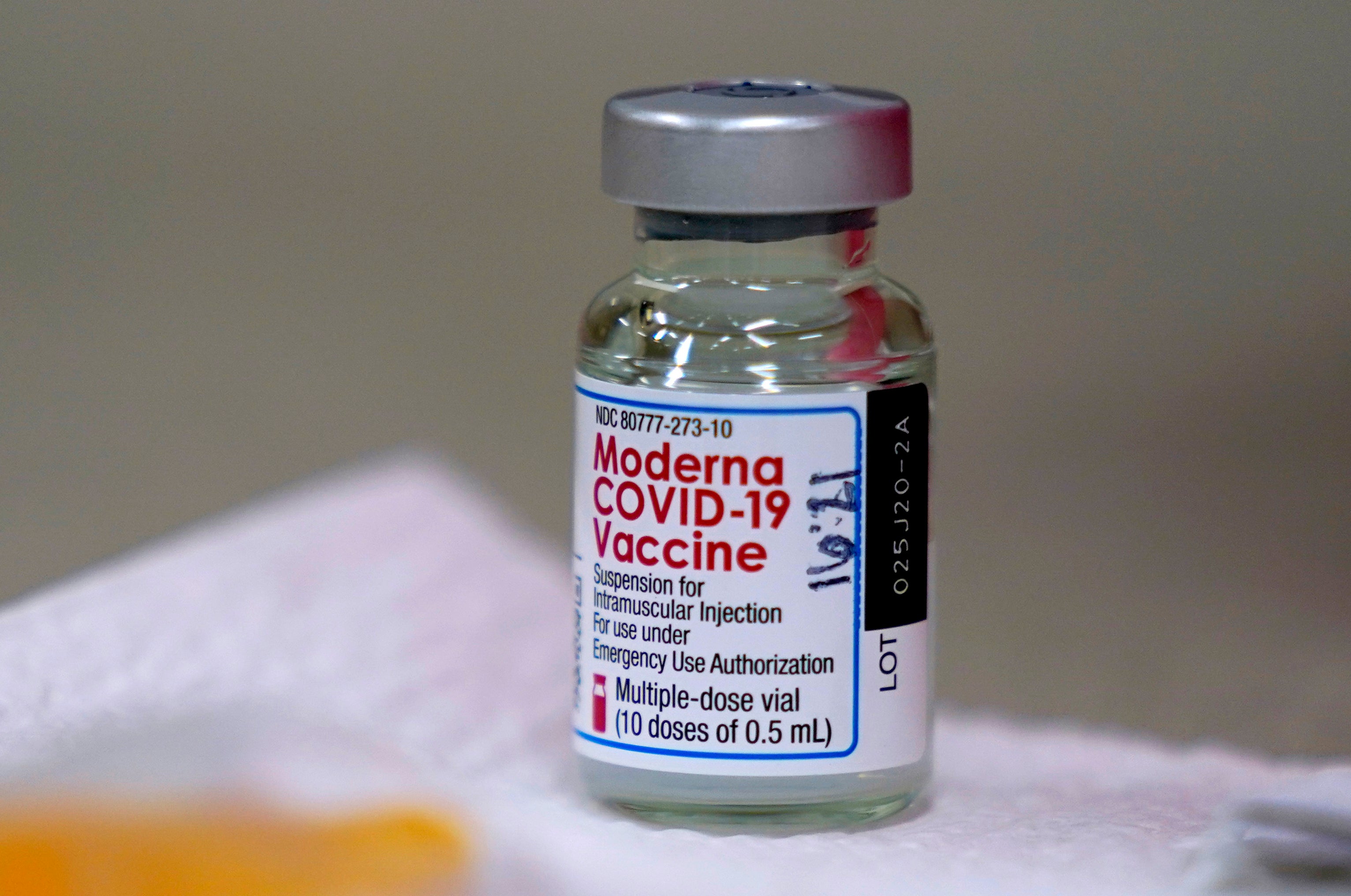
Your support makes all the difference.The Moderna Covid-19 vaccine has become the third to be approved by the UK.
The US pharmaceutical company’s jab was given the green light by Britain’s regulator and doses will be available in the spring.
The announcement comes as the rollout of the Pfizer and Oxford vaccines is scaled up to meet Boris Johnson’s target of immunising all care-home patients by the end of the month, with 1,000 vaccination centres expected to be operational by Sunday.
The government has also purchased an additional 10 million doses of the Moderna vaccine on top of its previous order of 7 million, taking the total to 17 million.
Supplies will begin to be delivered to the UK once Moderna expands its production capability, the Department of Health and Social Care said.
The Medicines and Healthcare products Regulatory Agency accepted the recommendation of the Commission on Human Medicines and authorised the Moderna vaccine following months of rigorous clinical trials and extensive analysis of the vaccine’s safety, quality and effectiveness.
The jab is 94 per cent effective in preventing disease, including in the elderly.
Matt Hancock, the health secretary, said: “This is fantastic news and another weapon in our arsenal to tame this awful disease.
“Through our vaccine delivery plan, we have already vaccinated nearly 1.5 million people across the UK.
“The Moderna vaccine will boost our vaccination programme even further once doses become available from the spring.
“While we immunise those most at risk from Covid, I urge everyone to continue following the rules to keep cases low to protect our loved ones.”
The authorisation comes just days after the end of the Brexit transition period, and two days after the European Medicines Agency (EMA) recommended granting a conditional marketing authorisation for the jab for adults.
As part of the transition period, until the end of December 2020, Covid-19 vaccine candidates authorised via the EMA would have automatically been valid in the UK.
The Department of Health and Social Care said the Moderna vaccine will be available for free and the government is working with the devolved administrations to ensure it is deployed fairly across the UK.
Like the other two vaccines, the Moderna vaccine will be deployed through hospital hubs for NHS and care staff and older patients to get vaccinated, through local community services with local teams and GPs, and through vaccination centres across the country.
Professor Jonathan Van-Tam, deputy chief medical officer for England, said: “The highly effective Moderna vaccine is another impressive success for science and is another testament to the hard work of researchers and selfless clinical trial volunteers.
“This vaccine will save lives once doses become available, but it is crucial we all continue to follow the rules to protect each other until enough people have been protected.”
Nearly 1.5 million people in the UK have already been vaccinated with the Pfizer-BioNTech and Oxford University-AstraZeneca vaccines.
The Joint Committee on Vaccination and Immunisation will submit updated advice on which groups to prioritise for vaccination before doses become available.
Additional reporting by PA



Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments