Johnson & Johnson: Single-dose vaccine approved for use in UK
Latest jab to be launched in UK has been shown to be 85% effective
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
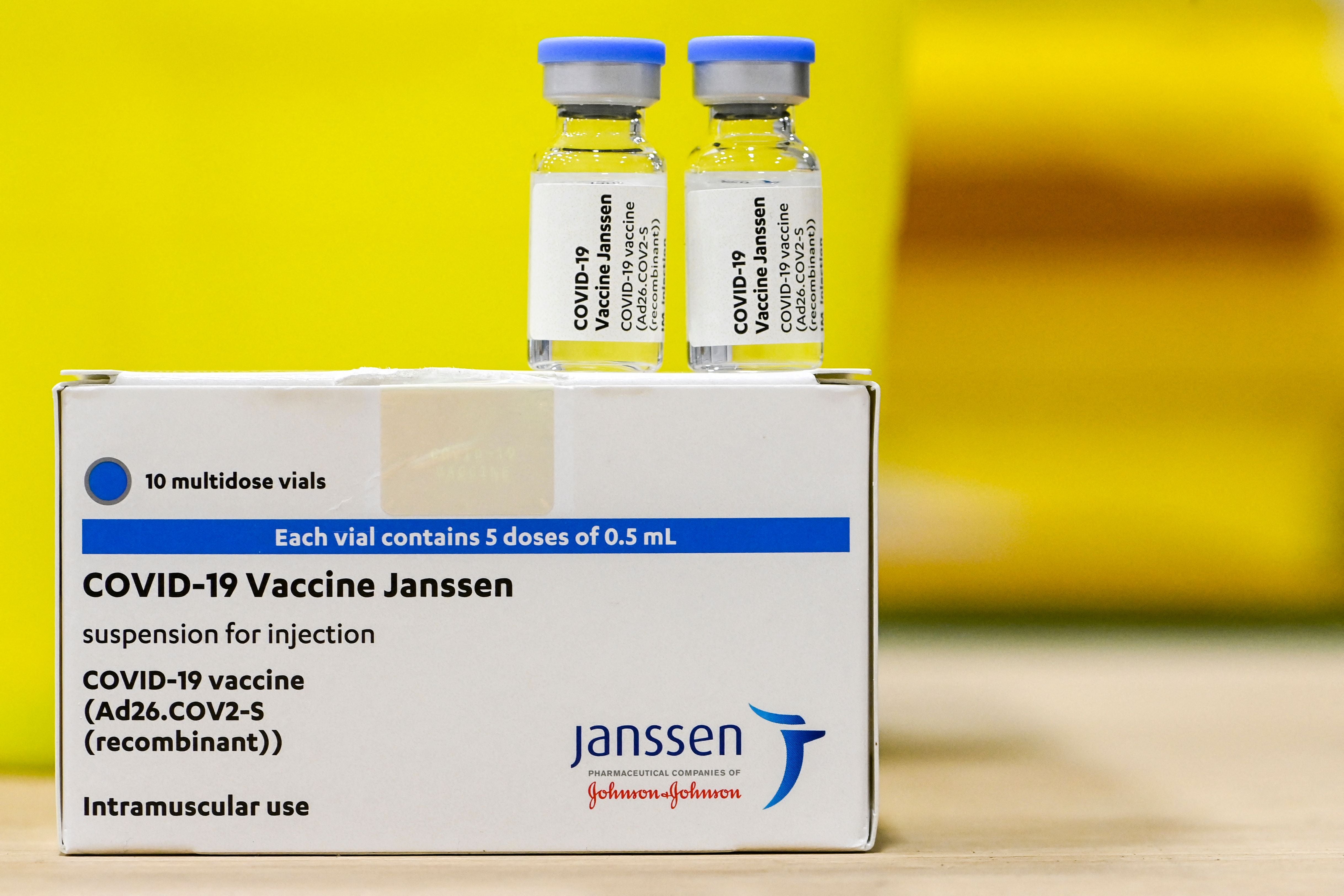
Your support makes all the difference.A single-dose coronavirus vaccine from Johnson & Johnson has been approved for use in the UK.
The jab, made by the US firm’s subsidiary Janssen, has shown to be 67 per cent effective overall at preventing moderate to severe Covid-19 and is thought to be 85 per cent effective in preventing severe disease or admission to hospital.
The government has ordered 20 million doses of the vaccine, amending a previous order for 30 million, and it is expected to be available in the UK towards the end of this year.
The “unprecedented scale and pace” of the vaccine programme rollout was behind the decision to cut the order, the government said.
Boris Johnson said the Medicines and Healthcare products Regulatory Agency (MHRA) approval of the safety of the vaccine was “very welcome news”, as he encouraged “everyone to get their jabs”.
Matt Hancock said it was “a further boost” to the UK’s successful vaccination programme.
“As Janssen is a single-dose vaccine, it will play an important role in the months to come as we redouble our efforts to encourage everyone to get their jabs and potentially begin a booster programme later this year,” the health secretary said.
Nadhim Zahawi, the vaccines minister, said: “The Janssen vaccine will be another weapon in our arsenal to beat this pandemic.”
The MHRA is thought to have held back from early approval of the vaccine after concerns were raised in the US about a link to extremely rare blood clots.
The clots are similar to those seen in a very small proportion of people having the Oxford-AstraZeneca jab.
In April, the European Medicines Agency said a warning about unusual blood clots with low blood platelet count should be added to the product information for the vaccine.
This followed eight cases of blood clots in more than 7 million people vaccinated in the US.
Belgium suspended the use of the vaccine for people under the age of 41 following a death from blood clots linked to the jab.
While many European countries are using the jab, Denmark has also dropped it due to concerns over side effects.
Johnson & Johnson has said the vaccine works across multiple variants of coronavirus.
In a clinical trial involving more than 40,000 people the level of protection against moderate to severe Covid-19 infection was found to be 72 per cent in the US arm of the trial.
It was 66 per cent in the Latin American trial, and 57 per cent in South Africa – where a variant of the virus has been dominating.
It is the fourth Covid-19 jab available for use in Britain and can be stored at normal fridge temperatures, which means it could be used for distribution in places such as care homes.
Jonathan Van-Tam, England’s deputy chief medical officer, has previously said the vaccine could be used for hard-to-reach groups of people, where recalling them for a second jab is not always successful.
The Joint Committee on Vaccination and Immunisation will now examine the data and provide guidance on who should receive it.
The jab is also part of the UK’s Cov-Boost study, which is looking at the effectiveness of a range of vaccines that could be used as a booster shot in the autumn.
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments