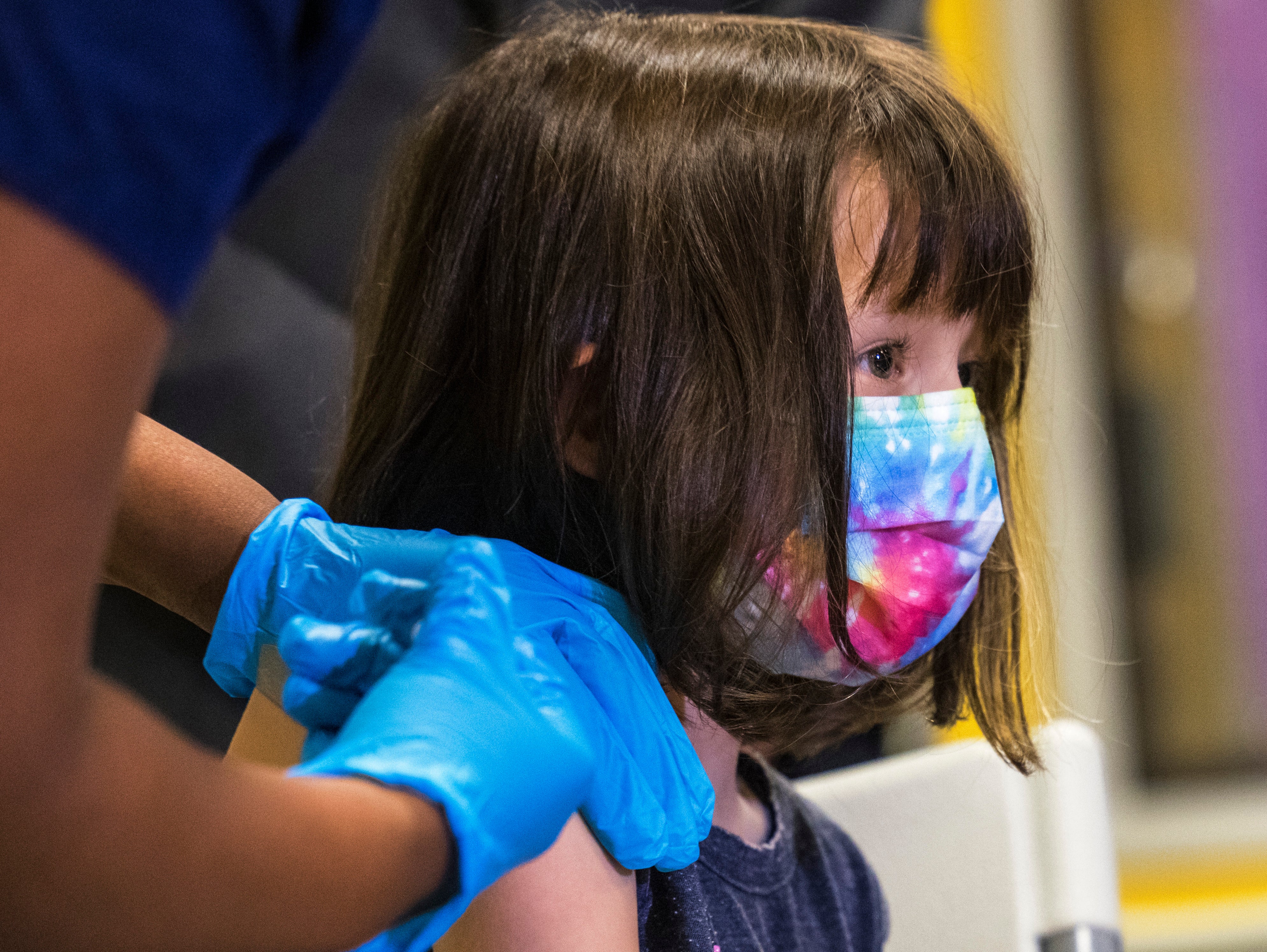
Covid: Vaccine decision for children as young as five expected next week
Exclusive: Review ‘moving at pace’ and expected to conclude within days
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.The UK’s medicines regulator is set to announce a decision on whether to vaccinate children aged five to 11 as early as next week, The Independent understands, with a rollout expected in the new year.
The Medicines and Healthcare products Regulatory Agency (MHRA) is “moving at pace” with its assessment, while a member of Britain’s vaccine watchdog said the group “won’t hang around” in making a recommendation to ministers once it’s concluded if the Pfizer vaccine is safe and effective to use in young children.
However, should the green light be given, it’s expected over-fives won’t start receiving the jab until the new year. The government has made clear that rolling out boosters and vaccinating the unprotected remains the priority – more so now with the threat posed by the omicron variant.
The Independent has been told that an announcement from the regulator’s review is now likely to be made next week.
One MHRA insider said the regulator was reluctant to commit to a date, but acknowledged that the rolling assessment had been accelerated. Another source close to the review said it was “moving at pace”.
A member of the JCVI, which will have any final say in recommending the use of vaccines among five to 11-year-olds, said “we won’t hang around” when the MHRA announces its decision.
The watchdog is already reviewing data on the risk posed by Covid to over-fives with asthma and deliberating whether this particular group should be prioritised as part of any vaccine rollout for young children.
“I do think that public opinion is shifting about vaccination of younger children which could widen our discussions,” said the JCVI member.
It still remains unclear when the government and NHS would look to start jabbing young children, with focus currently fixed on the vaccination of adults and the rollout of booster shots.
After approval was provided for the vaccination of 12 to 15-year-olds in September, it took just one week before children from this age group started receiving the jab.
However, amid concern posed by the new omicron variant and the government’s pledge to accelerate the rollout of boosters, the decision could be made by ministers and health officials to prioritise this pillar of the vaccination programme, before later turning attention to young children.
Last month, before the emergence of omicron, England’s deputy chief medical officer said that the vaccination of five to 11-year-olds is not a priority for the UK.
“Were it to be the case that the vaccine became licensed in five to 11-year-olds there would need to be a JCVI consideration of this point,” Professor Jonathan Van-Tam said.
“The big priority is actually the people who need the boosters, the partially vaccinated and the unvaccinated adults.”
Previous reports have suggested that it would not be until the spring before the rollout of vaccines for young children begins.
Azeem Majeed, a professor of primary care and public health at Imperial College London, said he did not expect those aged between five and 11 to start receiving their jab until the new year. “I would have gone a bit quicker with approving vaccines for children, both with those kids 12 and above and then those five to 11,” he added.
The EU has already approved the Pfizer jab for five to 11-year olds, and in the US more than 2.6 million children in this age group have received a first dose, according to White House estimates.
Announcing its decision late last month, the European Medicines Agency said: "The benefits … outweigh the risks, particularly in those with conditions that increase the risk of severe Covid-19.”
Pfizer and Moderna are meanwhile conducting vaccine trials for children as young as six months. Pfizer is testing a three-microgram dose, or one-tenth of its adult dose, in children between six months and five years of age.
Speaking at a Downing Street press conference on Monday, Dr June Raine, chief executive of the MHRA, said: “An application for use of the Pfizer vaccine in children aged five to 11 will be very, very carefully assessed as you would expect – for safety, for effectiveness at the proposed dose and for quality.”
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments