Pausing treatment of immunosuppressants ‘doubles response to Covid booster’
Interrupting the treatment of vulnerable people on the drugs could see a stronger immune response to the jab, a new study suggested.
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.Pausing the treatment of vulnerable people on immunosuppressants doubles their antibody response to the coronavirus booster vaccination, a new study has suggested.
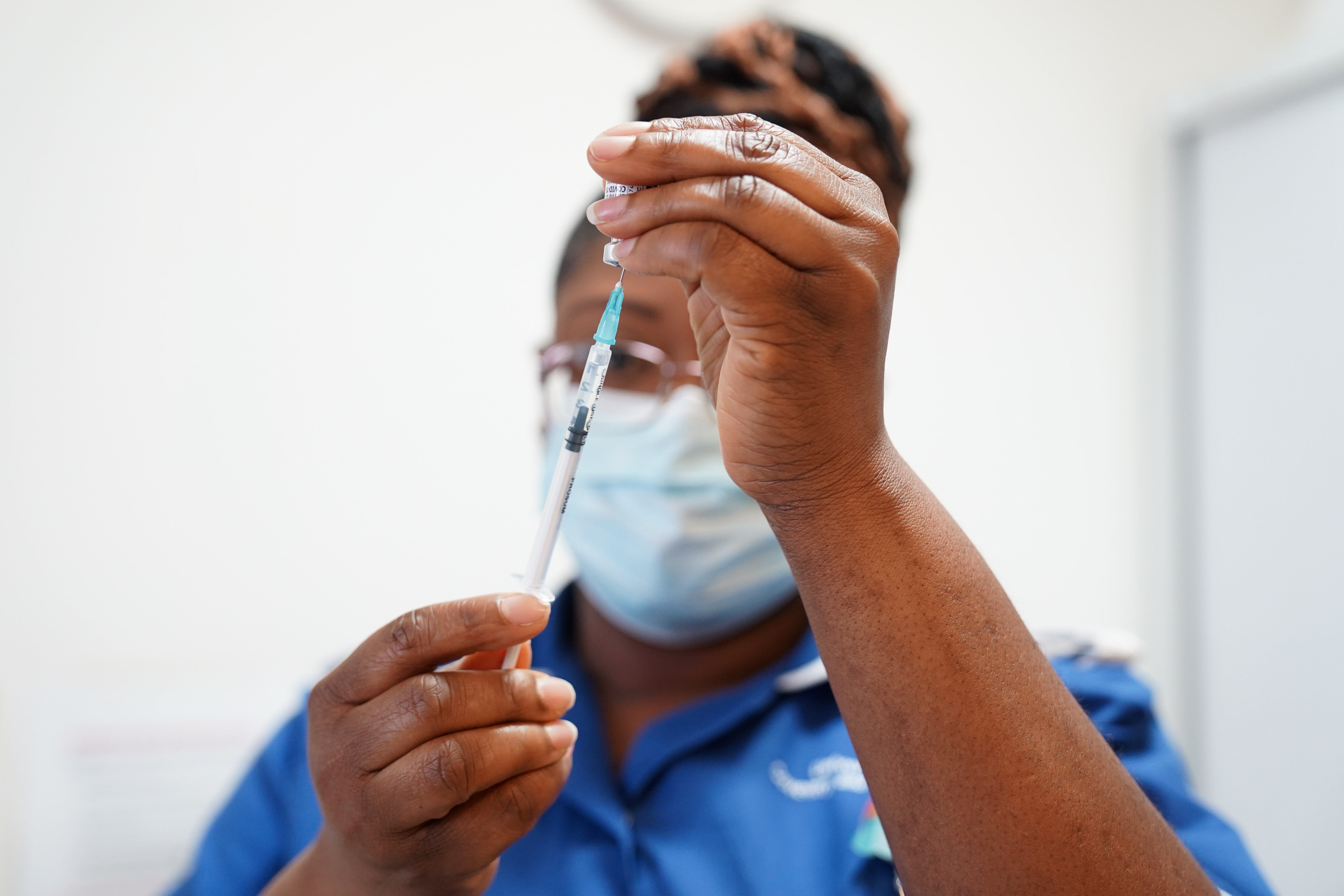
Researchers looked at the effect of interrupting treatment with methotrexate – prescribed for inflammatory conditions such as rheumatoid arthritis and skin conditions such as psoriasis – for two weeks after the jab.
The drug is the most commonly-used immune-suppressing drug, with about 1.3 million people in the UK prescribed it.
The main take-home message is that interrupting treatment for two weeks after Covid-19 B=booster vaccination doubles the immune response at week four and week 12 - sustained improvement in immune response
Many of them were among the 2.2 million clinically extremely vulnerable people advised to shield during the first phase of the Covid-19 pandemic, depending on specialist advice and on their risk factors.
Researchers found that after four weeks and 12 weeks, the spike-antibody level was more than two times higher in the group where the medication was suspended for two weeks following vaccination, compared to the group who continued use.
While there was a short-term increase in risk of flare-up of inflammatory conditions, most of these could be self-managed and did not put any pressure on the NHS, the study indicated.
Chief investigator Professor Abhishek Abhishek at the University of Nottingham and honorary consultant rheumatologist at Nottingham University Hospitals NHS Trust, said: “The main take-home message is that interrupting treatment for two weeks after Covid-19 booster vaccination doubles the immune response at week four and week 12 – sustained improvement in immune response.
“However, there is increased risk of disease flares in the four weeks after post vaccination, when the treatment is being suspended for two weeks.
“The increased risk of disease flares must be borne in mind, but most of these flares appear to be mild, not requiring NHS help and being self-managed.”
The study findings have been passed on to the Joint Committee on Vaccination and Immunisation (JCVI), which is considering the results and whether to make any recommendations.
Prof Abhishek said: “Implementing these results could vastly improve the protection provided by boosters against Covid-19 for millions of people living with these conditions.
“Covid-19 has left them vulnerable to serious illness whilst still having to live with the painful and troubling effects of their conditions.
“We hope this evidence is the next step in helping them with their lives going forward.”
The researchers cautioned that patients should not take matters into their own hands and instead consult with their doctor before making any changes to their treatment.
The study planned to recruit 560 patients but recruitment was stopped early by the independent study oversight committees when interim results from the first 254 participants showed a clear result.
During the trial, 127 participants were asked to suspend methotrexate use for two weeks and 127 to continue using it as usual, and spike-antibody levels in the two groups were compared.
The Vaccine Response On Off Methotrexate (Vroom) trial is funded by the National Institute for Health and Care Research and the Medical Research Council.
It was led by experts at the University of Nottingham in collaboration with colleagues from the University of Manchester, Imperial College London, the University of Oxford and Queen Mary University London.
The findings are published in Lancet Respiratory Medicine.
Subscribe to Independent Premium to bookmark this article
Want to bookmark your favourite articles and stories to read or reference later? Start your Independent Premium subscription today.