Thousands urged to try Covid antiviral treatment in new study
University of Oxford researchers hope to recruit further 6,000 volunteers for Panoramic trial
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.The government has urged the British public to take part in an antiviral drugs study designed to help the NHS treat coronavirus patients.
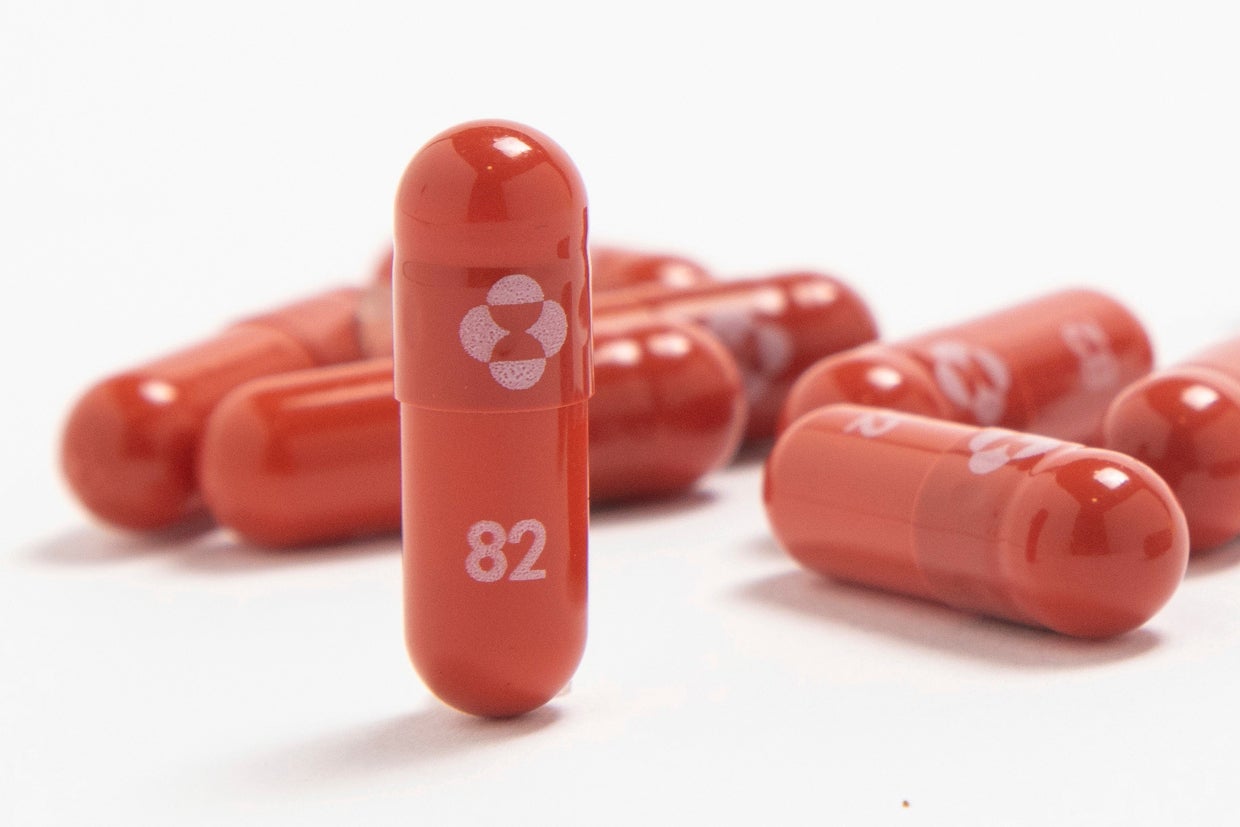
Volunteers will receive doses of molnupiravir, a drug that was approved by the UK’s Medicines and Healthcare products Regulatory Agency (MHRA) in early November. It was the first regulator to give it the green light.
The decision was taken after clinical research found the antiviral could reduce the risk of hospitalisation by around 30 per cent, with MHRA’s chief executive Dr June Raine describing the medicine as “another therapeutic to add to our armoury against Covid-19”.
Anyone over 50 can sign up to the current trial within five days of a positive Covid-19 test result. So can younger adults who are at heightened risk from the disease, due to pre-existing health conditions such as diabetes.
It is hoped that the study will allow the NHS to determine how the treatment programme can best be delivered to patients.
A total of 4,500 people have signed up so far, but experts from the University of Oxford want to enrol a further 6,000 volunteers.
Their plea was echoed by health secretary Sajid Javid, who said antivirals were “a vital part” of the UK’s strategy to live with Covid-19.
“If you’re eligible, please step forward for the Panoramic trial and play your part in a vital mission - helping us to learn more about medicines which could save thousands of lives,” he added.
Charities also backed the call, including the British Liver Trust, Cystic Fibrosis Trust, Diabetes UK and Kidney Care UK.
Following an initial order of 250,000 courses of molnupiravir, the government procured another 1.75 million from the manufacturer Merck Sharp & Dohme (MSD) in December.
At the same time, ministers also announced they had bought 2.5 million more courses of Pfizer’s Paxlovid antiviral drug, in what Mr Javid called a “mammoth deal”.
Speaking of the benefits of antivirals, Pippa Erskine, who suffers from cystic fibrosis and who has received a double lung transplant, said it was a “huge relief” to receive the treatment earlier this month when she contracted the virus.
“With restrictions easing, it’s so important that those vulnerable to Covid-19 have the best possible chance of staying protected against the virus and, most importantly, staying out of hospital,” Ms Erskine told the BBC.
Additional reporting by PA
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments