Coronavirus vaccine: Volunteers recruited as next phase of Oxford trial begins
Scientists want to test how older people and children respond in huge expansion of clinical tests
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
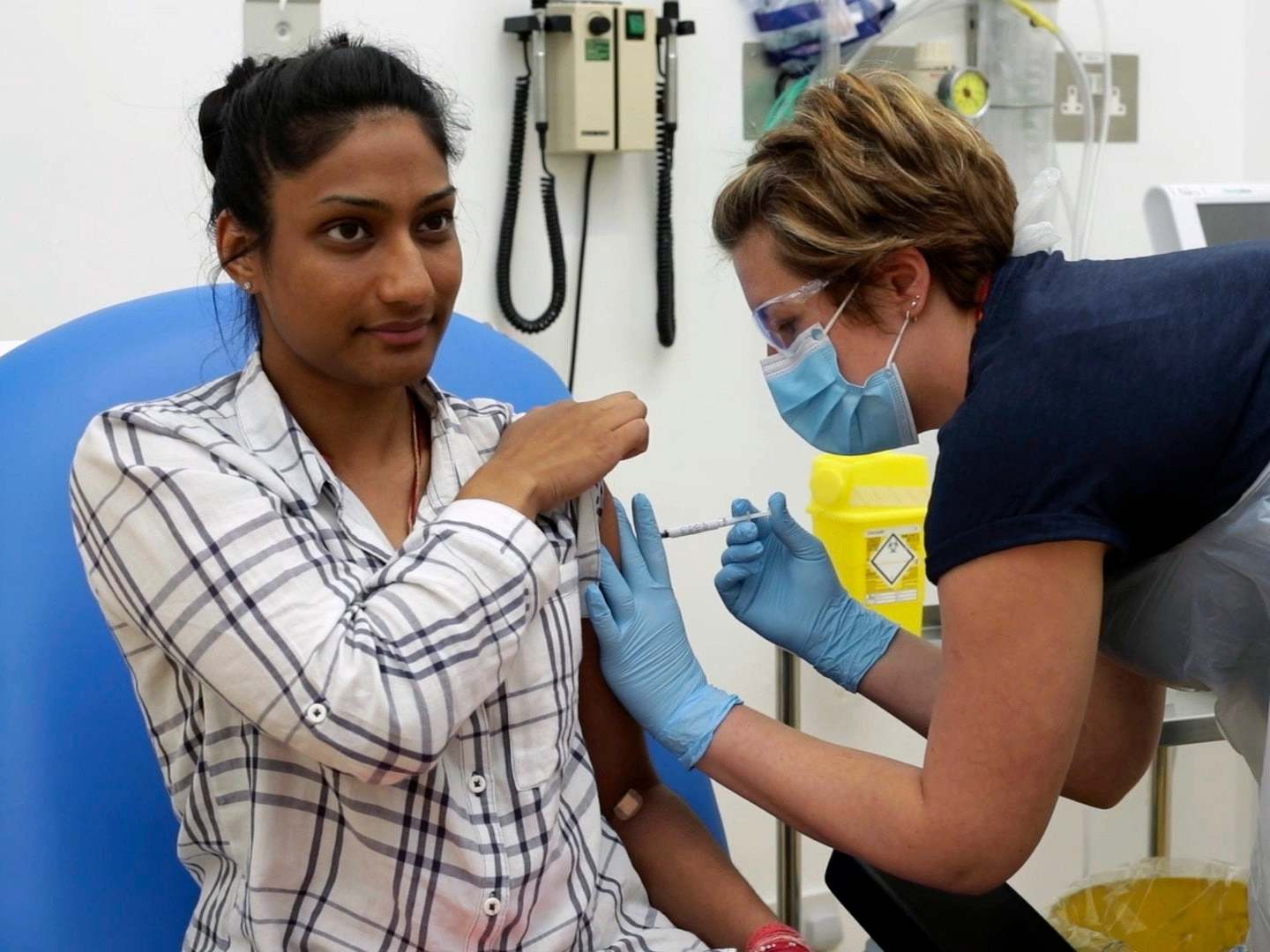
Your support makes all the difference.Researchers in the UK have begun recruiting volunteers for the next phases in clinical trials they hope could led to an effective coronavirus vaccine this year.
Work began in January on the vaccine, which uses a virus taken from chimpanzees and has been developed by the University of Oxford’s Jenner Institute and the Oxford Vaccine Group.
The first phase of trialling involved 160 healthy volunteers between 18 and 55. Now scientists want to recruit around 10,000 people for the second and third phases, a huge increase in volunteers which also involves expanding the age range.
Researchers want to assess how well the immune system responds in older people or children.
Professor Andrew Pollard, head of the Oxford Vaccine Group, said: “The clinical studies are progressing very well and we are now initiating studies to evaluate how well the vaccine induces immune responses in older adults, and to test whether it can provide protection in the wider population.”
Sarah Gilbert, professor of vaccinology at the Jenner Institute, said: “We have had a lot of interest already from people over the age of 55 years who were not eligible to take part in the phase I study, and we will now be able to include older age groups to continue the vaccine assessment.
“We will also be including more study sites, in different parts of the country.”
Saul Faust, professor of paediatric immunology and infectious diseases at the University of Southampton, which is also working on the trials, said: “We would now very much like to invite people from the Southampton area whose work brings them into possible contact with Covid patients or who are healthy and in the older age groups to take part in the next stage of trials of this Oxford Covid vaccine.
“This is one of only four vaccine trials underway worldwide and could pave the way for a vaccine to be delivered later this year.”
Initially, researchers are aiming to recruit up to 620 new volunteers in three categories.
Adult participants in the phase II and phase III groups will be randomised to receive one or two doses of either a vaccine known as ChAdOx1 nCoV-19, or a licensed vaccine (MenACWY) that will be used as a “control” for comparison.
The scientists said ChAdOx1 nCoV-19 is made from a weakened version of a common cold virus from chimpanzees that has been genetically changed to make it impossible for it to grow in humans.
This has been combined with genes that make proteins from the Covid-19 virus (SARS-CoV-2) which play a key role in the infection pathway of the SARS-CoV-2 virus.
Production of the vaccine has already been scaled up ahead of the trial to prepare as early as possible for potential future deployment.
AstraZeneca said this week it had the capacity to manufacture one billion doses of the University of Oxford’s potential Covid-19 vaccine and could begin supply in September. The pharmaceutical firm said it has secured the first agreements for at least 400 million doses of the vaccine.
In Scotland, the University of Glasgow will support the trials in collaborations with NHS Greater Glasgow and Clyde.
Dr Jennifer Armstrong, medical director of NHS Greater Glasgow and Clyde, said: “We’re proud to have NHS Greater Glasgow and Clyde join the global effort in finding an effective vaccine for Covid-19.”
Additional reporting by Press Association

Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments