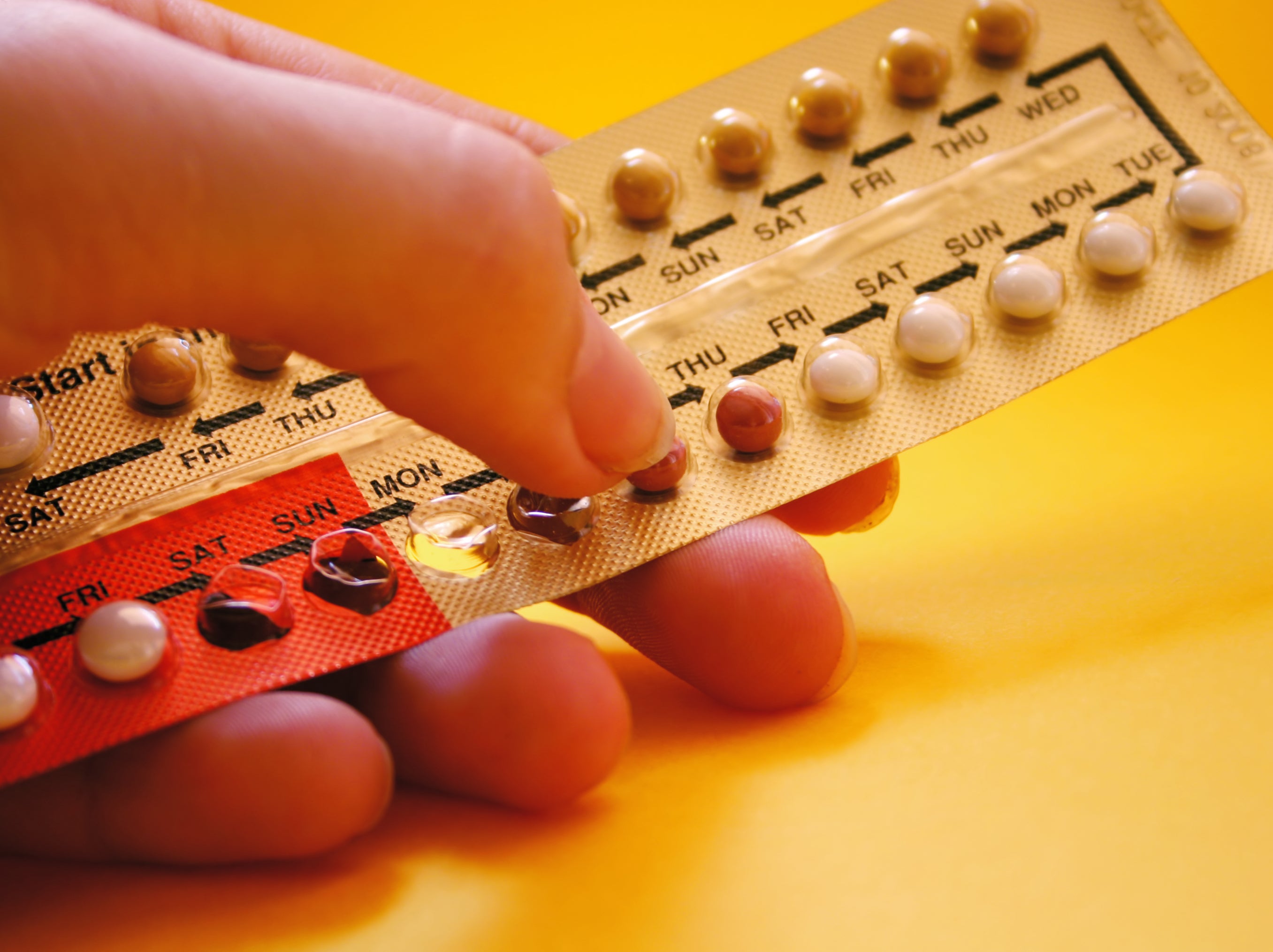
Contraceptive pill could become available over the counter
Women could be able to access the pill at pharmacies
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.Two versions of the contraceptive pill could be reclassified to allow the medication to be sold over the counter, the government has announced.
The Medicines and Healthcare products Regulatory Agency (MHRA) is set to launch a public consultation on whether to reclassify two progestogen-only contraceptive pills – Lovima 75 microgram film-coated tablets and Hana 75 microgram film-coated tablets, both of which contain desogestrel.
If the medicines are reclassified it would mark the first time in the nation’s history that daily contraceptive pills would be available over the counter at pharmacies.
A consultation is open until 5 March. Pills containing desogestrel will still be available from GPs and at sexual health clinics.
Dr Sarah Branch, director of vigilance and risk management of medicines at the MHRA, said: “Every response received will help us gain a better picture of whether people think the contraceptive pill with desogestrel should be available over the counter.
“We hope to hear from as many people and women’s groups as possible.”
The proposals were labelled a “positive step” by a consumer health care association.
Michelle Riddalls, chief executive of PAGB, the consumer healthcare association, said the body “fully [supports] these reclassification applications”.
She added: “The MHRA consultation represents a landmark opportunity in women’s health and one which we hope will be viewed positively.
“Both Maxwellia and HRA Pharma have asked the MHRA to permit the sale of their progestogen-only pill products under the supervision of a qualified pharmacist.
“As expert healthcare professionals, pharmacists are fully equipped to offer advice to anyone seeking information about over-the-counter medicines.”
PAGB hailed the applications’ “particular significance as they are the first to seek over-the-counter licences for any form of daily contraceptive pill, 60 years after the pill in its original form was made available via prescription on the NHS for married women only”.
Additional reporting by Press Association

Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments