Cancer drug developed over 20 years ago ‘could help patients living with chronic pain’
‘I just cannot sleep anymore because turning in bed hurts, my spine hurts laying down, and sitting up to sleep hurts even more,’ one bone cancer patient said
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.A drug developed over 20 years ago to treat cancer could help patients living with crippling pain, according to new research.
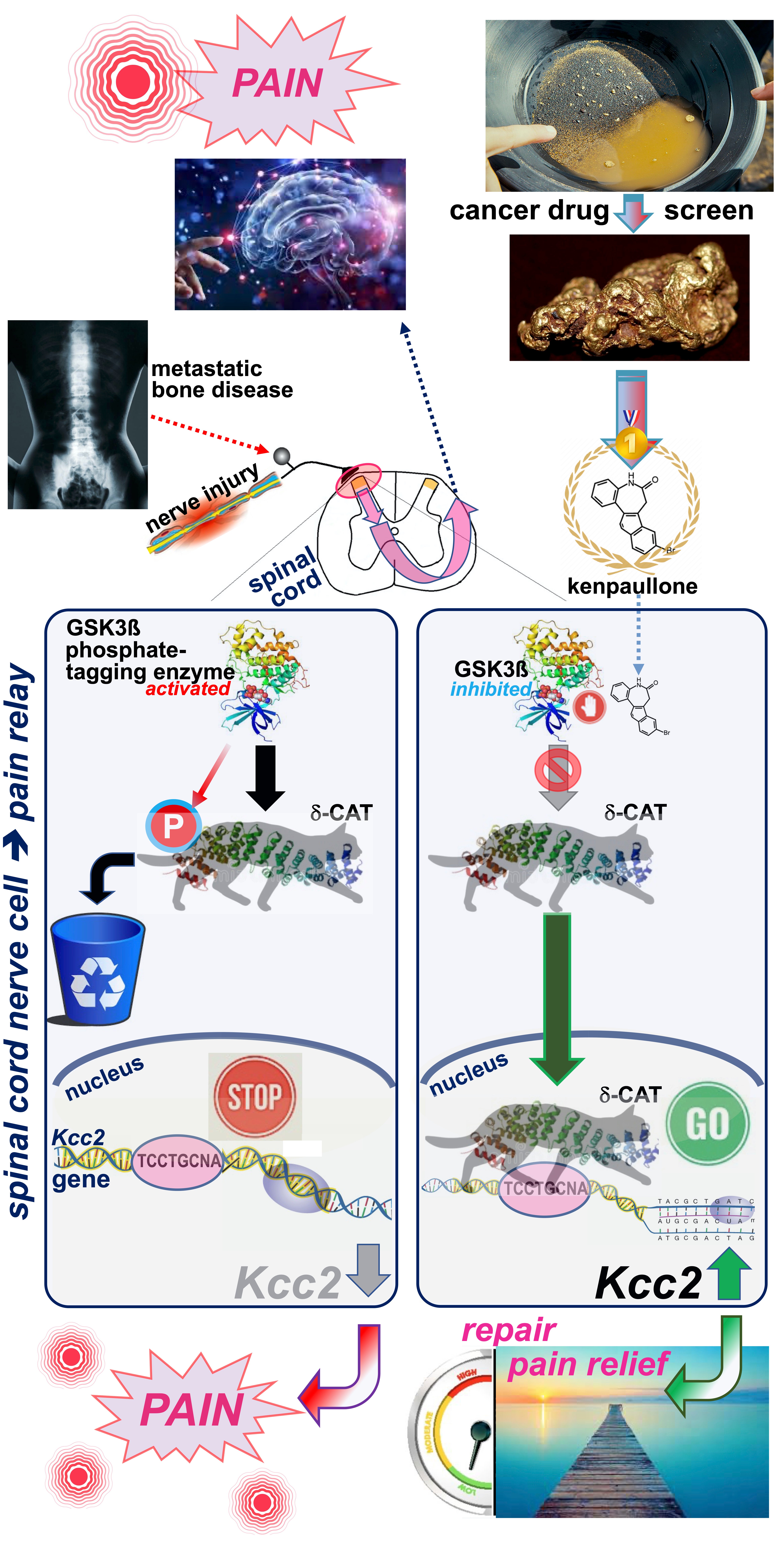
Kenpaullone switches on a gene that douses chronic inflammation, say scientists.
Experiments on mice and humans found it was remarkably successful at alleviating nerve injury and bone tumour symptoms.
The US team is hopeful clinical trials will see equally successful results in humans suffering a host of conditions.
Up to 8 million people in the UK live with chronic pain. Major causes include arthritis and spine damage.
Lead author Professor Wolfgang Liedtke said: “New drugs and other therapies against chronic pain need to be safe, i.e., the fewer side effects the better.
“It’s especially important they be non-addictive and non-sedative, while being effective against nerve injury pain and cancer pain, preferably with a minimal time to official approval.
“Because chronic pain, like many chronic diseases, has an important root in genetic switches being reprogrammed in a bad way, a disease modifying treatment for chronic pain should reset the genetic switches, not just cover up the pain, as with opioid and aspirin/Tylenol-like painkillers.”
Prof Liedtke practiced pain medicine for the last 17 years at Duke University in North Carolina and is now an executive at Regeneron Pharmaceuticals in New York.
Globally, about 1.5 billion people suffer from chronic pain. It affects an estimated 50 million in the US.
One bone cancer patient said: “I just cannot sleep anymore because turning in bed hurts, my spine hurts laying down, and sitting up to sleep hurts even more.
“During daytime, I have constant brain fog, interrupted by pain that within minutes gets worse 10-out-of-10, against a background of constant burning pain which gets worse toward the afternoon and evening.
“I hurt more when I go to the bathroom. The pain medication makes my brain fog worse. I feel like a zombie. I am badly constipated and itch all over.”
Similarly, those with chronic nerve injury damage caused by diabetes, side effects of medication or severe shingles describe their lives as misery.
Prof Liedtke and colleagues sought better treatments by surveying the “junkyard of cancer drugs” with the potential to be repurposed.
They tested over 1,000 compounds contained in two libraries of the National Cancer Institute.
In addition to stopping cells multiply, many reset maladaptive genetic switches in non-dividing nerve cells.
In mice with bone cancer, Kenpaullone was found to stop pain caused by nerve constriction.
It triggered a gene called Kcc2, which expels chloride from neurons. Low levels silence signalling between cells - reducing pain.
In all forms of chronic pain studied in experimental animals and human spinal cord models, Kcc2 disappeared from neurons making up the primary pain gate.
Prof Liedtke said: “Kenpaullone is an effective analgesic for chronic and challenging-to-treat pain, such as that caused by nerve injury and bone cancer. It reprograms pathways.
“The pain relief was profound, long-lasting and with a protracted onset, consistent with the drug having an impact on gene regulation.
“At this stage, we knew we had met the basic requirement of our screen of shelved cancer drugs, namely identified Kcc2 gene expression-enhancers, and demonstrated they are analgesics in valid pre-clinical pain models.”
Further tests yielded “resoundingly affirmative results”. They showed Kenpaullone affects spinal cord processing of pain.
It also reduces nerve injury-induced elevation of chloride levels in pain-relaying neurons.
The researchers discovered the underlying mechanism. Kenpaullone inhibits a pain relaying enzyme called GSK3-beta that turns off a gene called delta-CAT.
Prof Liedtke added: “These findings suggest Kenpaullone and similarly-acting kinase-inhibitory compounds, as well as delta-CAT gene therapy, have the potential to become new tools in our toolbox against chronic refractory pain, including nerve injury pain and cancer bone pain, and likely against other forms of chronic pain associated with low Kcc2 expression.
“This approach may also be effective against other neurologic and psychiatric disorders in which this mechanism appears to contribute to the disease.”
The study, published in the journal Nature Communications, identified three other highly promising drugs that could carry out similar functions.
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments