FDA OKs 1st targeted drug for common lung cancer mutation
U.S. regulators have approved the first medicine for patients with the most common type of lung cancer whose tumors have a genetic mutation long considered untreatable with drugs
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.U.S. regulators have approved the first medicine for patients with the most common type of lung cancer whose tumors have a genetic mutation long considered untreatable with drugs.
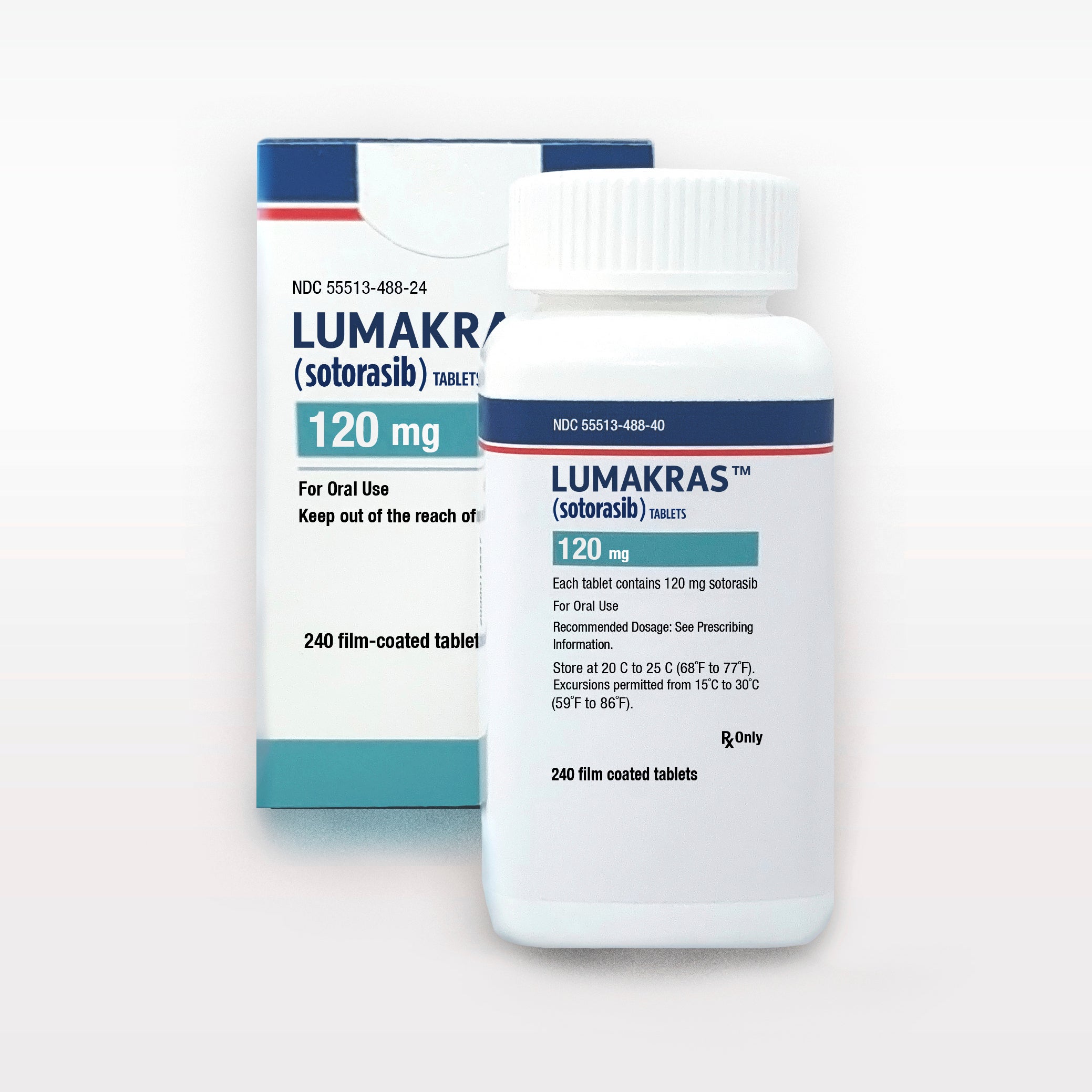
The Food and Drug Administration on Friday said it has approved Amgen’s drug Lumakras to treat non-small cell lung cancer with the mutation in patients who have worsened after initial treatment with at least one other drug. Each year, about 13,000 U.S. patients are diagnosed with this cancer and mutation.
This is the first targeted therapy for tumors with a so-called KRAS mutation, the FDA noted. This type of mutation occurs in genes that help regulate cell growth and division. The mutation is involved in many cancer types.
“Today’s approval represents a significant step toward a future where more patients will have a personalized treatment approach,” Dr. Richard Pazdur, director of the FDA’s Oncology Center of Excellence, said in a statement.
Amgen said Lumakras, also known as sotorasib, will cost $17,900 per month, though most patients will pay less, depending on health insurance and other factors. Lung cancer is the most common cancer type and is the leading cause of cancer deaths in the U.S.
The agency also approved diagnostic tests from two companies that can determine if patients have the specific mutation, known as KRAS G12C, targeted by the drug.
Amgen and other drugmakers are working to develop several medicines designed to attack tumors with KRAS mutations.
“KRAS has challenged cancer researchers for more than 40 years,” Dr. David M. Reese, Amgen’s head of research and development, said in a statement.
The FDA approved the drug on an accelerated schedule, based only on early study results, because of its potential and the lack of options for these patients. It is requiring further testing to confirm the drug’s benefit.
In a study including 124 patients, 36% had their tumors shrink or disappear. Improvements lasted for six months or longer for nearly 60% of those who benefited.
Common side effects included diarrhea, joint and muscle pain, fatigue and liver damage. The FDA said the drug should be discontinued if patients develop liver damage or a type of lung disease.
___
Follow Linda A. Johnson at https://twitter.com/LindaJ_onPharma.
___
The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute’s Department of Science Education. The AP is solely responsible for all content.