EU drug regulator recommends clearing vaccine for monkeypox
The European Medicines Agency says the smallpox vaccine made by Bavarian Nordic should also be authorized against monkeypox as an outbreak of the once-rare disease sickens people across Europe
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.The European Medicines Agency said Friday that the smallpox vaccine made by Bavarian Nordic should also be authorized against monkeypox, as the outbreak of the once-rare disease continues to sicken people across the continent.
The European Union drug regulator said its recommendation was based on animal studies that suggest the vaccine protects non-human primates from monkeypox. It is up to the EU's executive arm, the European Commission, to formally approve the vaccine based on the EMA's recommendation.
“To confirm the effectiveness of the vaccine against monkeypox, the company will collect data from an observational study that will be carried out during the ongoing monkeypox outbreak in Europe,” the EMA said. It added that the vaccine's safety profile was “favorable” and the benefits of its use during the ongoing monkeypox outbreak outweighed the risks, noting mostly mild to moderate side effects.
The vaccine, known as Imvanex in Europe but sold as Jynneos in the United States, was already cleared for use against monkeypox by American regulators.
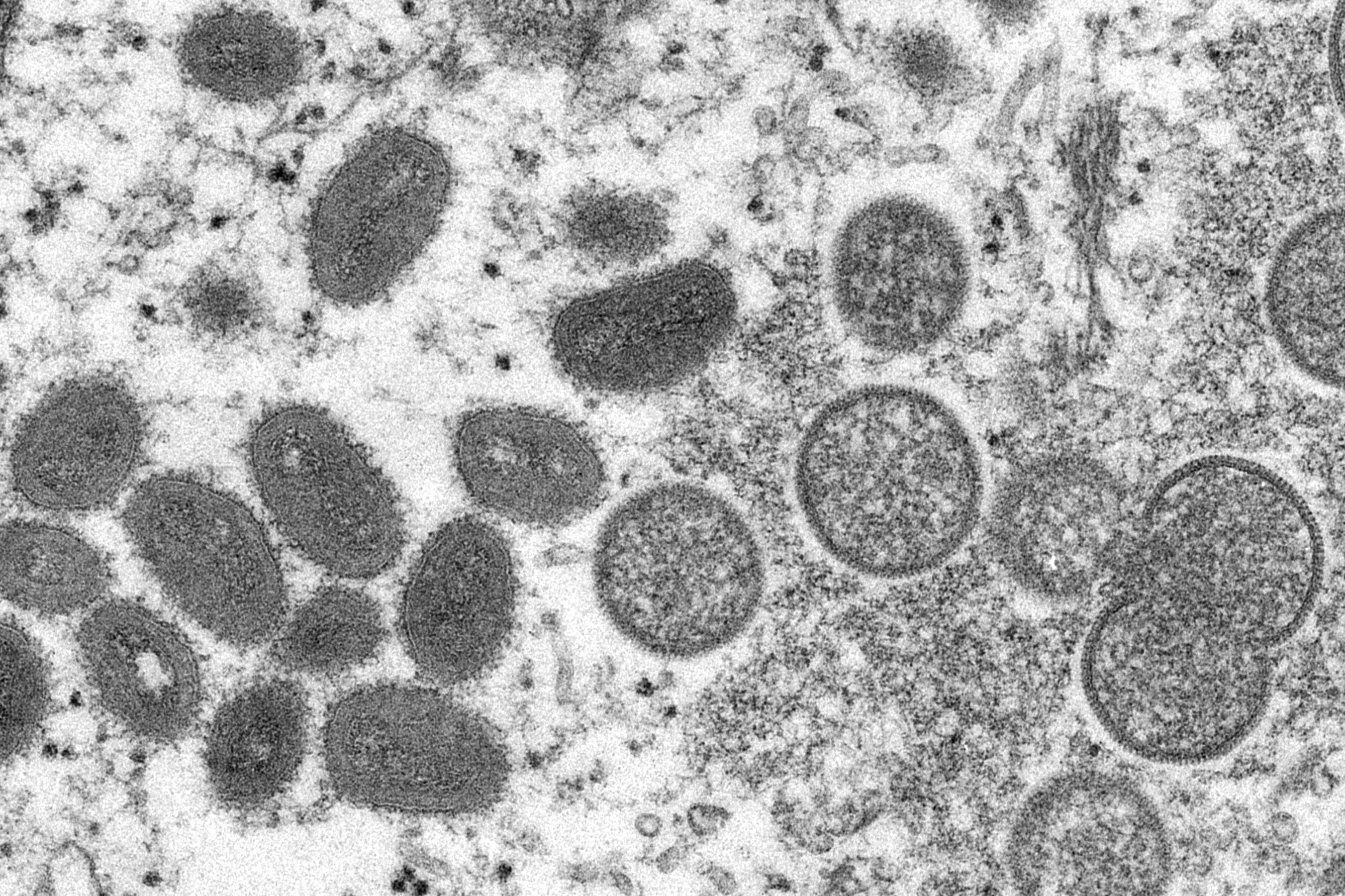
Of the more than 15,000 monkeypox cases reported worldwide, nearly 70% are in Europe, with more than 30 countries across the continent affected.
Doses of the Bavarian Nordic vaccine are extremely limited. Most of the world's supply has already been bought by countries and regions including Britain, Canada, the EU and the U.S. None have gone to Africa, where a more severe version of monkeypox has killed dozens of people. No monkeypox deaths have been reported in rich countries.
People who catch monkeypox often experience symptoms that include fever, body aches, a rash and lesions; most recover within weeks without needing medical attention.
Authorities in numerous countries, including Britain, Germany and the U.S., have offered the vaccine to health workers and those at high risk of being infected by monkeypox.
In the U.S., soaring demand for the monkeypox vaccine caused the appointment system to crash in New York City, one of many places where supplies have run out almost immediately after they arrived.
The World Health Organization is deciding whether or not to declare the outbreak to be a global emergency, after convening its experts for a meeting on Thursday.