Live Updates: Israel trials 4th dose of COVID-19 vaccine
Israel has begun trials of a fourth dose of coronavirus vaccine in what is believed to be the first study of its kind
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
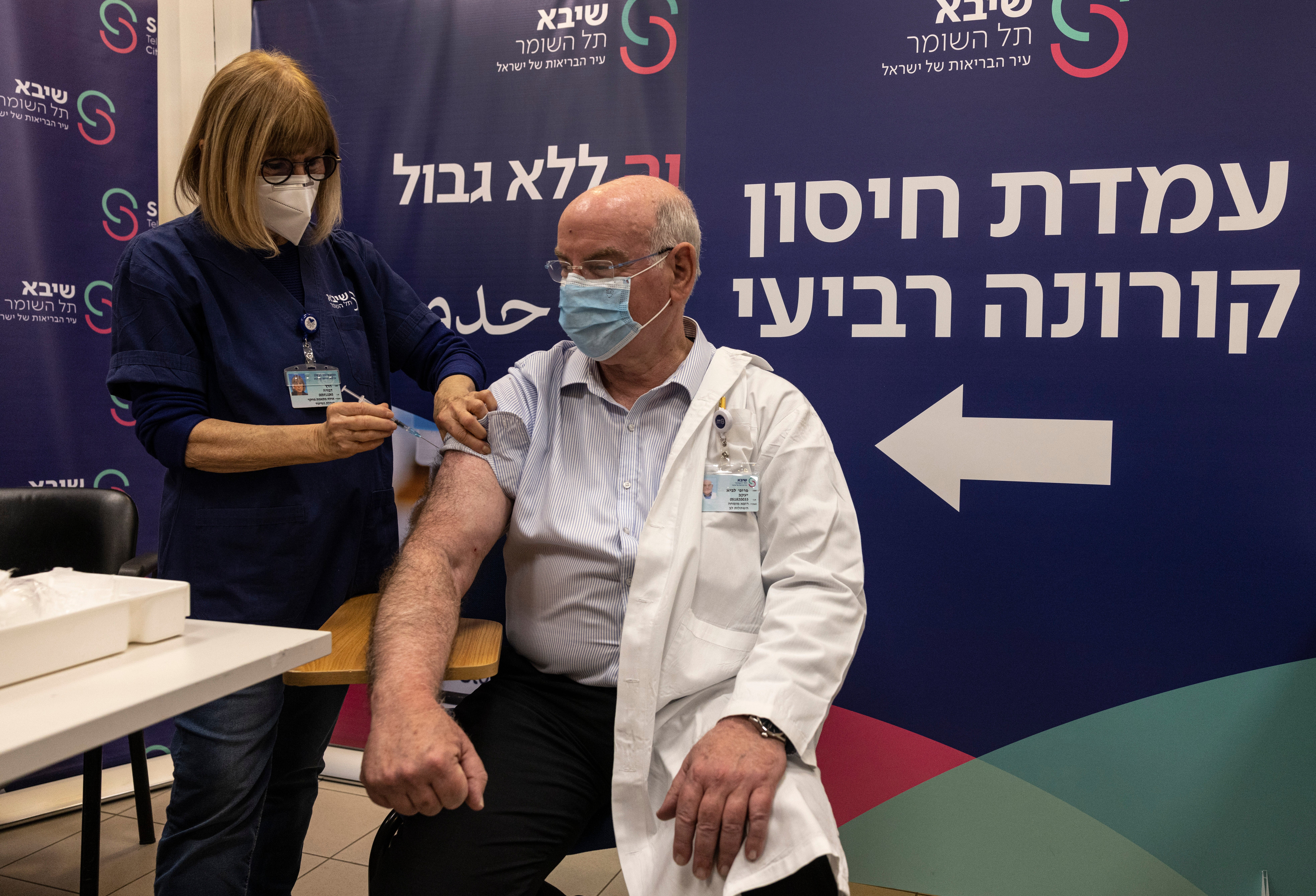
Your support makes all the difference.JERUSALEM — Israel has begun trials of a fourth dose of coronavirus vaccine in what is believed to be the first study of its kind.
The trial began at Sheba Medical Center, outside Tel Aviv with 150 medical personnel who received a booster dose in August receiving a fourth shot of the Pfizer BioNTech vaccine. The staff receiving the additional dose were tested and found to have low antibody levels.
The trial came as Israeli officials have considered rolling out a second tranche of booster shots to its population as the country grapples with rising infections with the new omicron variant.
Prof. Jacob Lavee, former director of the heart transplant unit at Sheba, said “hopefully, we’ll be able to show here… that this fourth booster really provides protection against the omicron, which is highly needed.”
Israel rolled out a world-leading vaccination campaign early this year. Just over 4.2 million of Israel’s 9.3 million people have received a third dose of the Pfizer/BioNTech vaccine.
Israel has recorded at least 8,242 deaths from coronavirus since the start of the pandemic.
___
HERE’S WHAT YOU NEED TO KNOW TODAY ABOUT THE CORONAVIRUS PANDEMIC:
Delta flight to Shanghai turned back because of COVID rules.
Variant disrupts holiday travel but not shopping.
France sees over 100,000 daily infections for the 1st time
Go to https://APNews.com/coronavirus-pandemic for updates throughout the day.
___
HERE’S WHAT ELSE IS HAPPENING TODAY:
BERLIN — Police in southern Germany say eight people were arrested after violence during a protest by opponents of coronavirus restrictions and vaccinations.
Eight officers were punched or kicked as groups of people tried to break though police chains set up to prevent the protesters marching in the Bavarian town of Schweinfurt on Sunday evening, police said. Officers used batons and pepper spray. Police said a 4-year-old child whose mother was among those trying to break through the barricades briefly had to be given medical attention after coming into contact with pepper spray.
Demonstrations against restrictions and a planned universal vaccine mandate have flared up in Germany at times in recent weeks.
Authorities are in the process of implementing restrictions agreed last week to slow the spread of the new omicron variant. Four more states were introducing curbs Monday that include capping the number of people at private gatherings at 10, excluding children under 14.
___
SEOUL, South Korea — South Korea says it has granted an emergency authorization to Pfizer’s COVID-19 treatment pill, Paxlovid.
The Ministry of Food and Drug Safety said Monday it expects the introduction of Paxlovid to help diversify options for COVID-19 treatments and prevent patients’ conditions from becoming serious amid surging infections and critical cases in South Korea.
The Korea Disease Control and Prevention Agency says the government has signed contracts to procure Paxlovid pills enough to cover 362,000 patients. It says the Paxlovid pills will be delivered to South Korea as early as mid-January.
It says South Korea has also signed contracts to procure Merck’s COVID-19 pills to treat 242,000 people.
The drug safety agency says it’s still reviewing whether to approve Merck’s molnupiravir.