Researchers working on world’s only testosterone patch for menopause
Women seeking treatment for the effects of menopause on libido currently cannot be prescribed testosterone on the NHS.
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
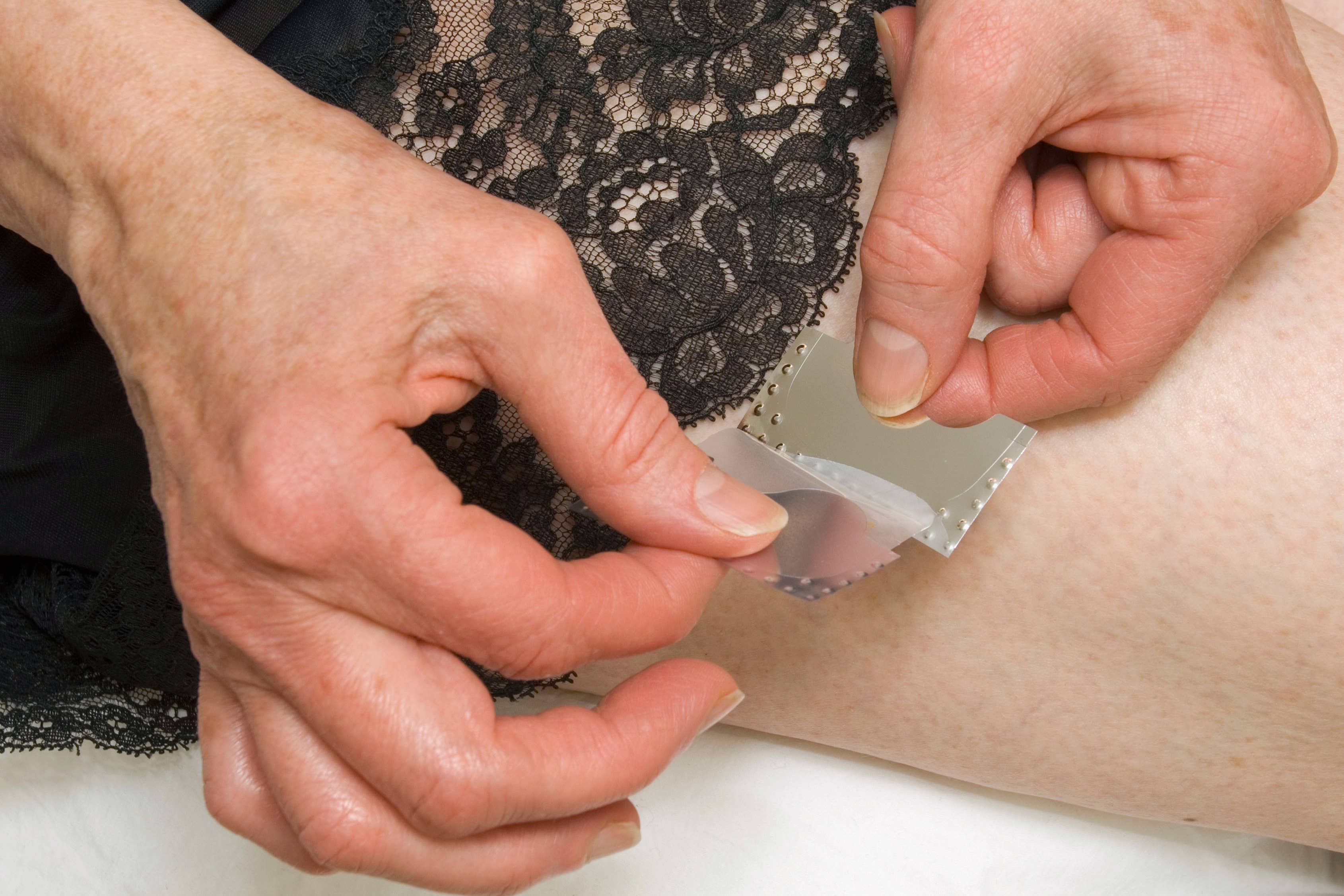
Your support makes all the difference.Researchers are developing the world’s only testosterone patch for women with menopausal symptoms.
Medherant, a company founded by the University of Warwick’s Professor David Haddleton, is aiming to start clinical trials in the autumn.
If clinical trials go well, and the treatment gets regulatory approval, this would be the only testosterone replacement patch available globally and would be introduced first in the UK.
Haddleton said the potential to improve women’s lives is “huge”, helping them with their loss of sex drive.
This is a very exciting development for us – the potential of this technology to improve women’s lives is huge
Women seeking treatment for the effects of menopause on libido currently cannot be prescribed testosterone on the NHS.
Some resort to irregular doses of gel only approved for use on men, experts say.
Testosterone is an essential hormone for women and its production drops heavily after menopause.
Although oestrogen and progesterone hormone replacement therapy (HRT) patches – which stick to the skin to deliver medications – are available, there is no testosterone delivery patch for women suffering adverse symptoms from the menopause.
Haddleton said: “This is a very exciting development for us – the potential of this technology to improve women’s lives is huge.
“The work we’re doing at Medherant and at Warwick isn’t just theoretical, but instead aimed at a problem women are facing which can drastically affect their everyday lives and jobs.”
“This could deliver a product that is much needed and is just not available.
“With the technology already proven to work we can use our new patch to remove needless misery from women’s daily lives.
“We hope this will transform life for women suffering from post-menopause issues nationally and indeed globally.”
Since 2015, guidelines issued by the National Institute for Health and Care Excellence (Nice) have recommended that testosterone supplementation be considered for menopausal women with low sexual desire if HRT alone is not effective.
The new patch is intended to address this gap in menopause products, providing treatment specifically for women that can be made widely available.
John Burt, chief executive of Medherant, which raised almost £3 million for the study, said: “Having the funding in place for the first clinical trial of our testosterone patch for post-menopausal women will enable Medherant to take a major step towards registration of the product and being able to address this significant gap in the options available for women in this very important stage of their lives.”