Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
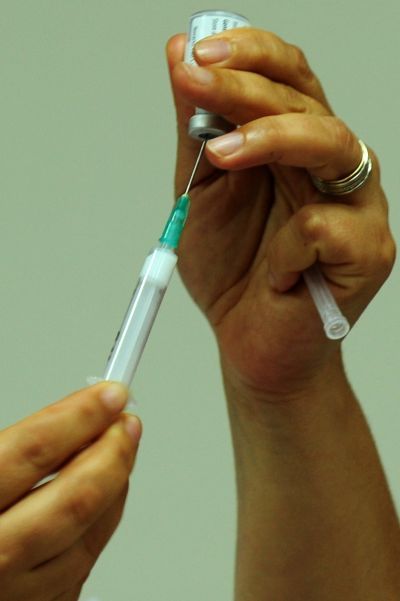
Your support makes all the difference.Amgen's Prolia, a new shot designed to help postmenopausal females with osteoporosis, received US Food and Drug Administration (FDA) approval on June 1.
The FDA's announcement explained, "the safety and efficacy of Prolia in the treatment of postmenopausal osteoporosis was demonstrated in a three-year, randomized, double-blind, placebo-controlled trial of 7,808 postmenopausal women ages 60 to 91 years. In the study, Prolia reduced the incidence of vertebral, non-vertebral, and hip fractures in postmenopausal women with osteoporosis."
Julie Beitz, MD, the director of the FDA's Office of Drug Evaluation III, said, "due to its prevalence, osteoporosis is a serious concern to public health. The approval of Prolia provides another treatment option for postmenopausal women with osteoporosis who are susceptible to fractures."
Those involved in the trial reported side effects including "back pain, pain in the extremities, musculoskeletal pain, high cholesterol levels, and urinary bladder infections. Serious adverse reactions include hypocalcaemia (low calcium levels in the blood), serious infections, including infections of the skin, and dermatologic reactions such as dermatitis, rashes, and eczema."
However, "Prolia works to decrease the destruction of bone and increase bone mass and strength." According to National Public Radio (NPR), a nonprofit membership US-based media organization, "an earlier test comparing Prolia, known generically as denosumab, with the popular osteoporosis pill Fosamax, or alendronate, gave a slight edge on bone-strengthening to Prolia. Side effects were similar."
Prolia is to be injected every six months opposed to taking alendronate pills once a week. If needles freak you out, don't worry, you can still reach for Fosamax and save a good deal of money. Prolia is expected to cost $1650 (€1350.46) or two shots opposed to $400 (€327.38) for a year's supply of Fosamax.
For more information on osteoporosis, go to: http://www.niams.nih.gov/Health_Info/Bone/Osteoporosis/osteoporosis_ff.asp
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments