Hysteroscopic sterilisation is a 'serious safety concern,' researchers say
Around 1,500 women a year in the UK undergo the procedure, which involves inserting permanent birth-control implants
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.A “serious safety concern” has been raised by researchers over a form of sterilisation for women, which involves blocking the fallopian tubes with a titanium metal implant.
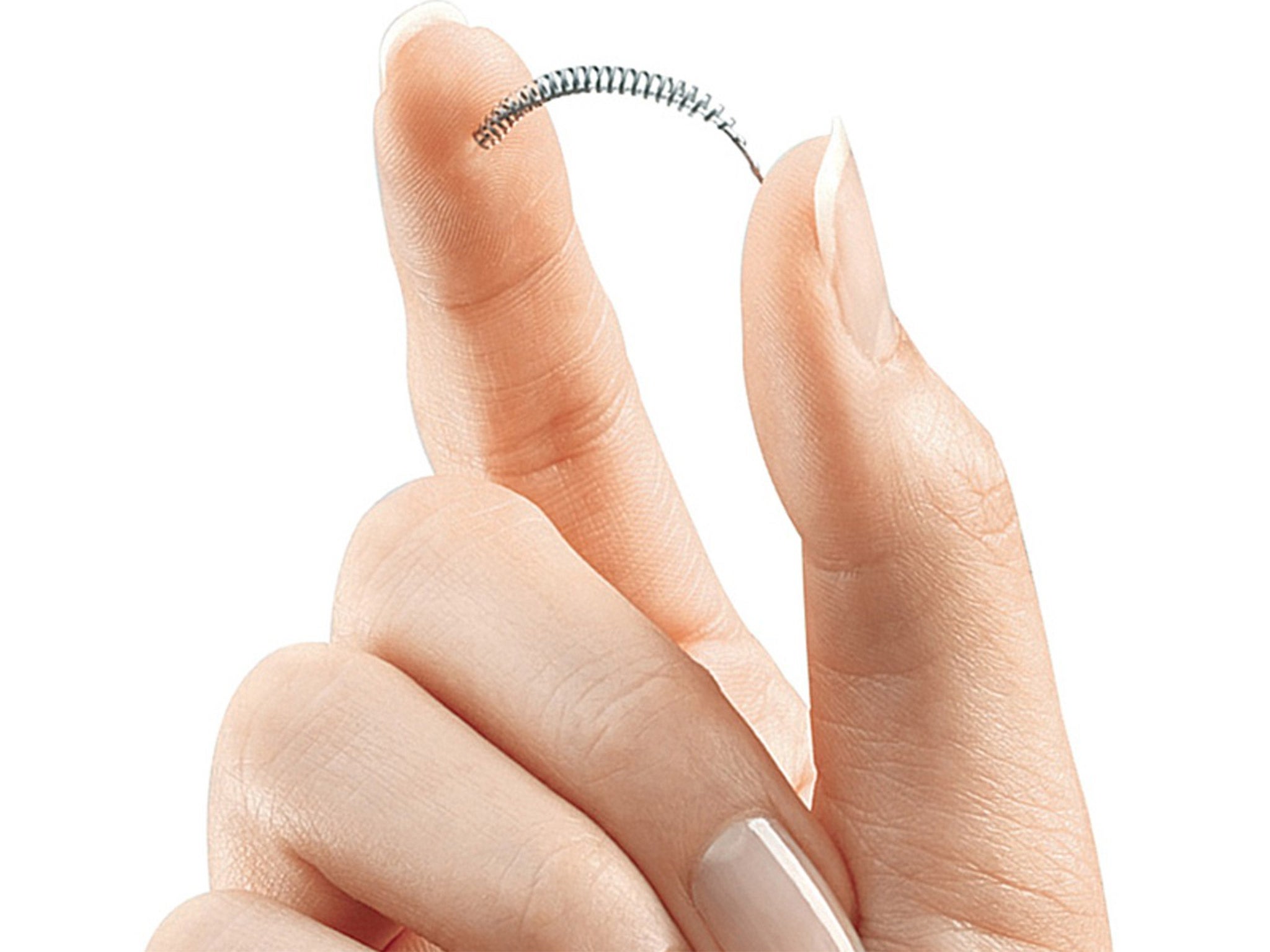
Around 1,500 women a year in the UK undergo the procedure, known as hysteroscopic sterilisation, or HS in which permanent birth control implants are inserted using local anaesthetic. The implant, known as an Essure device, causes the fallopian tube to form scar tissue around it, which eventually blocks the tube.
Essure was first developed by a firm called Conceptus and won ‘pre-market approval’ (PMA) by the US Food and Drug Administration (FDA) in 2002. The device has been dogged by controversy ever since Conceptus was taken over by German pharmaceutical firm Bayer two years ago with a campaign initially led by environmental activist Erin Brockovich.
The FDA is now investigating allegations from several women who say their reports of severe pain were buried by researchers and thousands of women have registered complaints about discomfort and internal injuries they say are related to the device.
Now a team of researchers has carried out the first study to compare the performance, safety and other outcomes of hysteroscopic sterilisation with laparoscopic sterilisation, also known as tubal occlusion when the fallopian tubes are blocked using clips or rings.
Under HS a narrow tube with a telescope at the end, called a hysteroscope, is passed through the patient’s vagina and cervix. A guidewire is used to insert a tiny piece of titanium metal into the hysteroscope, then into each of the fallopian tubes meaning the surgeon does not need to cut into the body.
They are the two most common forms of female sterilisation used by millions of women worldwide.
The study, published in the BMJ this week, found that women who use HS have a significantly higher risk of needing repeat operations compared to those using the alternative method.
The study looked at more than 8,000 women undergoing HS and more than 44,000 women undergoing laparoscopic sterilisation between 2005 and 2013 in New York State.
Researchers discovered that women choosing HS have a similar risk of unintended pregnancy but a more than 10-fold higher risk of undergoing reoperation, defined as repeated sterilisation, in the first year following surgery - equivalent to around 21 additional reoperations per 1,000 patients undergoing surgery.
1,500
The number of women undergoing HS a year in the UK
Women were eight times more likely to undergo a reoperation at two years and six times more likely at three years following the initial sterilisation procedure. Reported complications related to inserting Essure included pelvic pain, haemorrhage, and device migration or incompatibility.
Art Sedrakyan, the study’s lead researcher and Professor of Healthcare Policy and Research at the Weill Medical College of Cornell University, told The Independent: “We hope women will take to time and weigh the benefits and harms of the two procedures, discuss their specific situation and comorbid conditions with their physician and make informed decisions.”
A spokeswoman for the Medicines and Healthcare products Regulatory Agency (MHRA) said: “We currently have no information to suggest that Essure devices used in the UK are unsafe to use.”
A statement from Bayer said: “Essure is a highly effective birth control option with a positive benefit-risk profile for women who have completed their families and want permanent contraception with a non-surgical procedure.
“Over a decade of research and development and a decade of real world experience supports the safety and efficacy of Essure.”
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments