'Transformative' HIV prevention pill to be offered on the NHS from September
'This is a pivotal moment in the fight against HIV,' say campaigners after High Court ruling forced NHS bosses to act
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.A “transformative” HIV prevention drug will be made available on the NHS from September after a High Court battle.
NHS England said the pre-exposure prophylaxis (PrEP) treatment will be provided to an initial 10,000 people via a three-year trial.
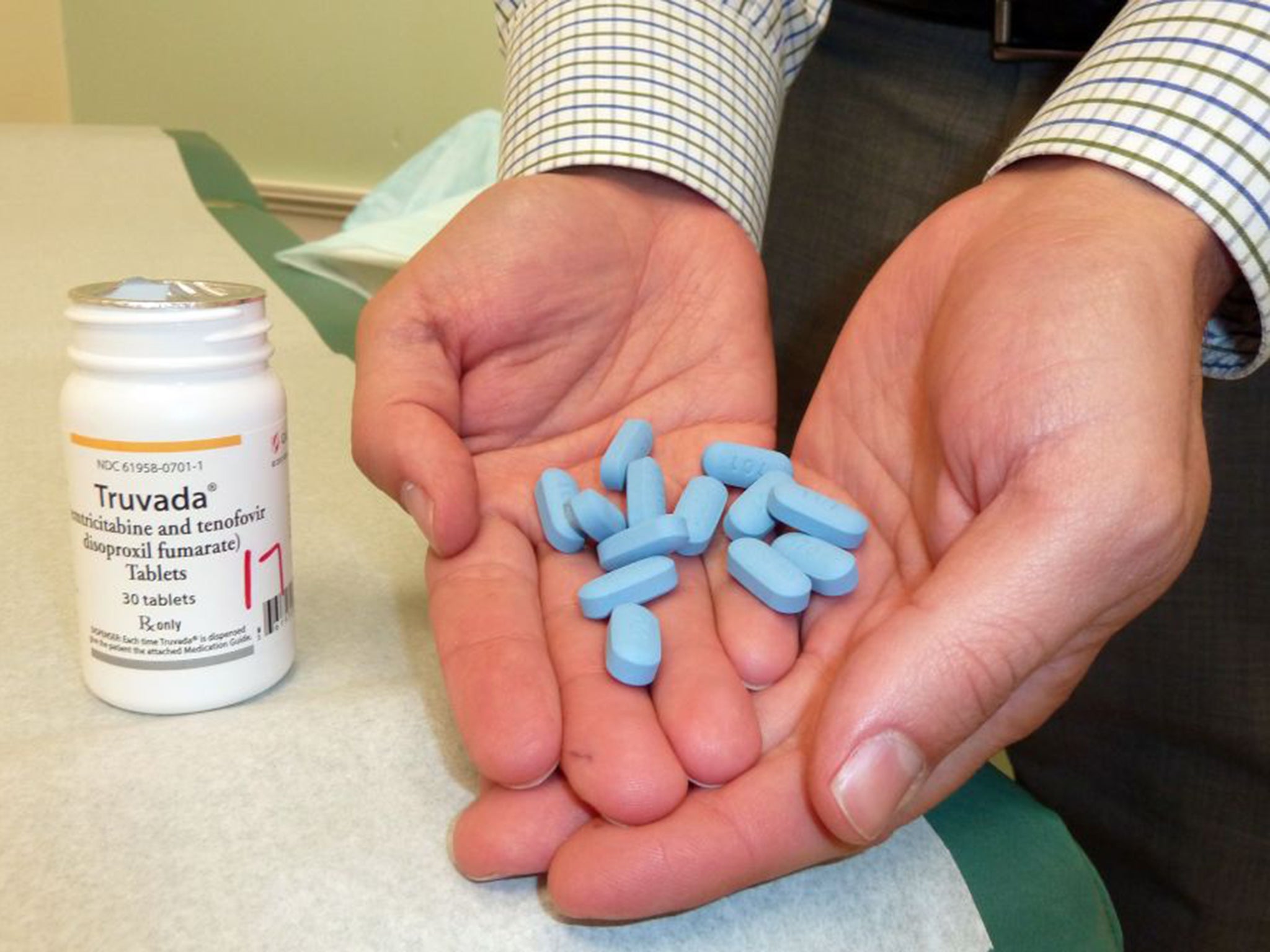
It comes in the form of a pill, called Truvada, that is taken before sex and has been shown to reduce the chance of contracting HIV in high-risk individuals by around 86 per cent.
The decision follows a High Court ruling that the NHS should pay for PrEP despite its insistence that the burden should fall on local authorities. The verdict was later upheld by the Court of Appeal.
PrEP will be offered through sexual health clinics in London, Brighton, Manchester, Liverpool and Sheffield from early September, with more clinics joining in October and the full trial being in place by April 2018. Priority will be given to people particularly at risk, including those whose partner may be HIV positive.
NHS England will spend a total of £10m on the trial.
Simon Stevens, chief executive of NHS England, hailed the introduction of the drug as a “major new intervention” in the battle against HIV/Aids.
He said it "should complement and supercharge the wide-ranging and increasingly successful effort to prevent HIV”.
“It’s another milestone in more than three decade’s worth of progress in tackling one of humanity’s major health challenges.”
Deborah Gold, chief executive of the National AIDS Trust, which was involved in the High Court challenge, said: ““This is a pivotal moment in the fight against HIV. PrEP, if targeted properly at those in need and at risk, offers the possibility of transforming the English HIV epidemic.
“From September, people at high risk of HIV will have access via this NHS-funded trial in England to an empowering new tool that is truly individually controlled and not subject to negotiation with a partner, leading to the improvement of many, many lives. We warmly welcome this announcement.”
However, some campaigners said the trial should be brought forward so it is fully in place before next April.
Ian Green, chief executive of sexual health charity Terrence Higgins Trust, said: "We’re pleased that NHS England has announced a start date for the much anticipated PrEP trial. This PrEP trial has been gaining momentum in England, and is vital as we work towards ending HIV transmissions across the UK.
"The priority must now be to make sure that the trial reaches everyone at risk of HIV, and that it is rolled out speedily across the whole country, by the end of this year at the very latest. Spring 2018 is not soon enough.
"Now that the PrEP trial drug has been procured, we’re well on the way to protecting over 10,000 people at risk of HIV. To make sure no-one at risk of HIV is left behind, it is crucial that at the end of this trial in three years time, a clear process for routinely commissioning PrEP on the NHS is agreed."
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments