Breast cancer drug too expensive to be routinely available on NHS, watchdog rules
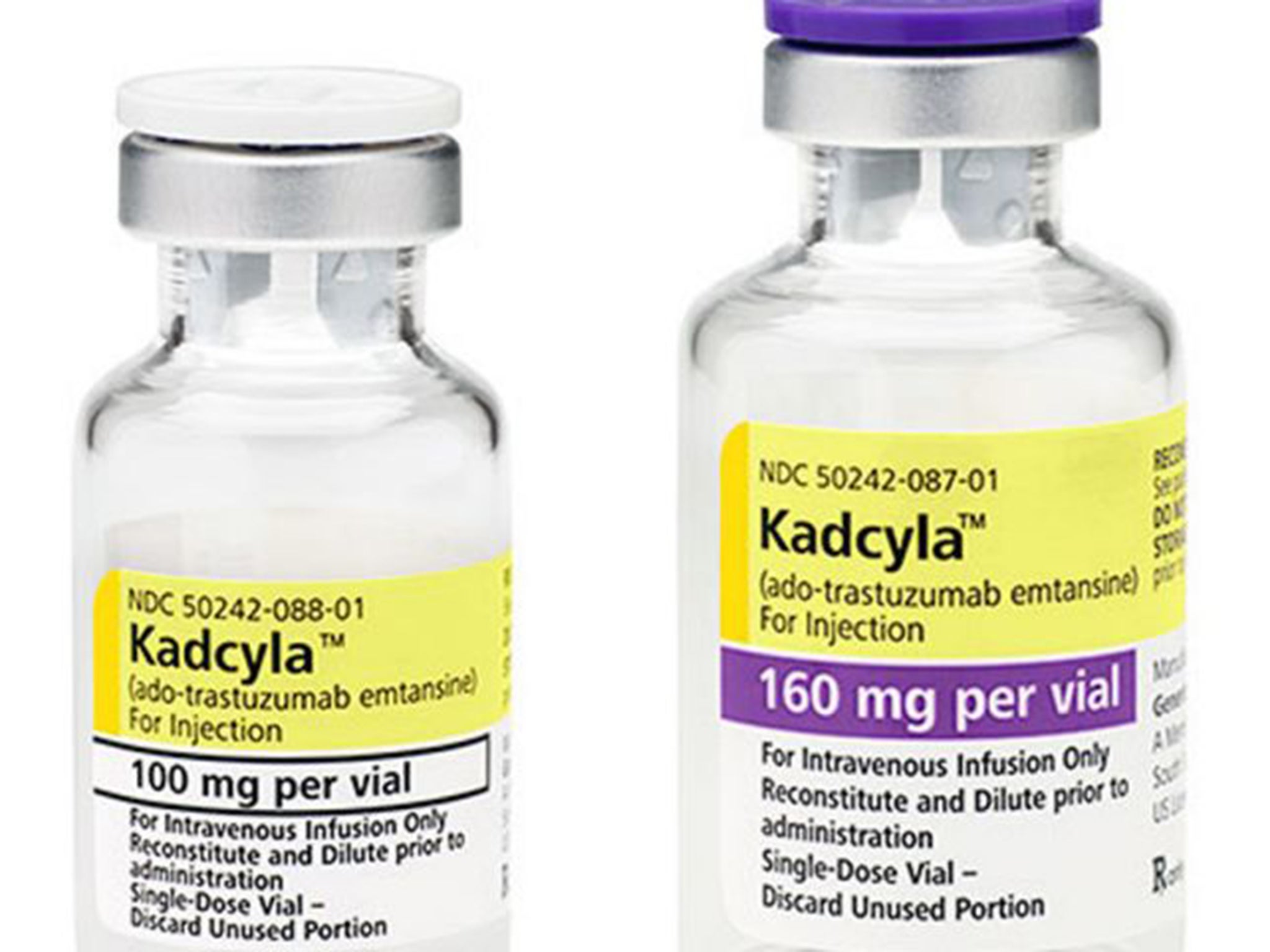
The pioneering drug Kadcyla can give women dying from an aggressive form of breast cancer extra months of life
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.A pioneering breast cancer treatment will not be routinely available on the NHS because its price is “too high”, a watchdog has ruled.
The National Institute for Health and Care Excellence published final draft guidance saying Kadcyla – which can give women dying from an aggressive form of breast cancer extra months of life – was not set at an affordable price.
The manufacturer Roche offered a discount, but it was not enough to sway Nice to recommend the drug across England.
The drug will still be available in England through the Cancer Drugs Fund for some patients.
In Scotland, the organisation that decides which medicines should be routinely available on the NHS chose not to approve the drug last year.
Nice said Roche had agreed a “significant” discount with NHS England to stop the drug being removed from the CDF, but a smaller discount had been offered to Nice for the drug’s more widespread use.
This means women will need to ask their oncologist to apply to the CDF for funding for Kadcyla.
It is licensed for HER2-positive breast cancer and has been shown to extend life by almost six months on average in women who have tried other treatments, although some patients live much longer.
Sir Andrew Dillon, chief executive of Nice, said: “We recognise that Kadcyla has a place in treating some patients with advanced breast cancer. However, the price that the manufacturer is asking the NHS to pay in the long term is too high.
“Despite a growing public campaign for a fair deal on the cost of important new cancer medicines, it is disappointing that there appears to have been no meaningful attempt by Roche to reconsider its price to secure Kadcyla’s long-term future in the NHS, outside of the Cancer Drugs Fund.”
Dr Caitlin Barrand, assistant director of policy and campaigns at Breast Cancer Now, said it was “hugely disappointing news”.
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments