New drug therapy ‘highly effective’ for young children with severe eczema
Dupilumab already licensed in UK for adults and children aged six to 18
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
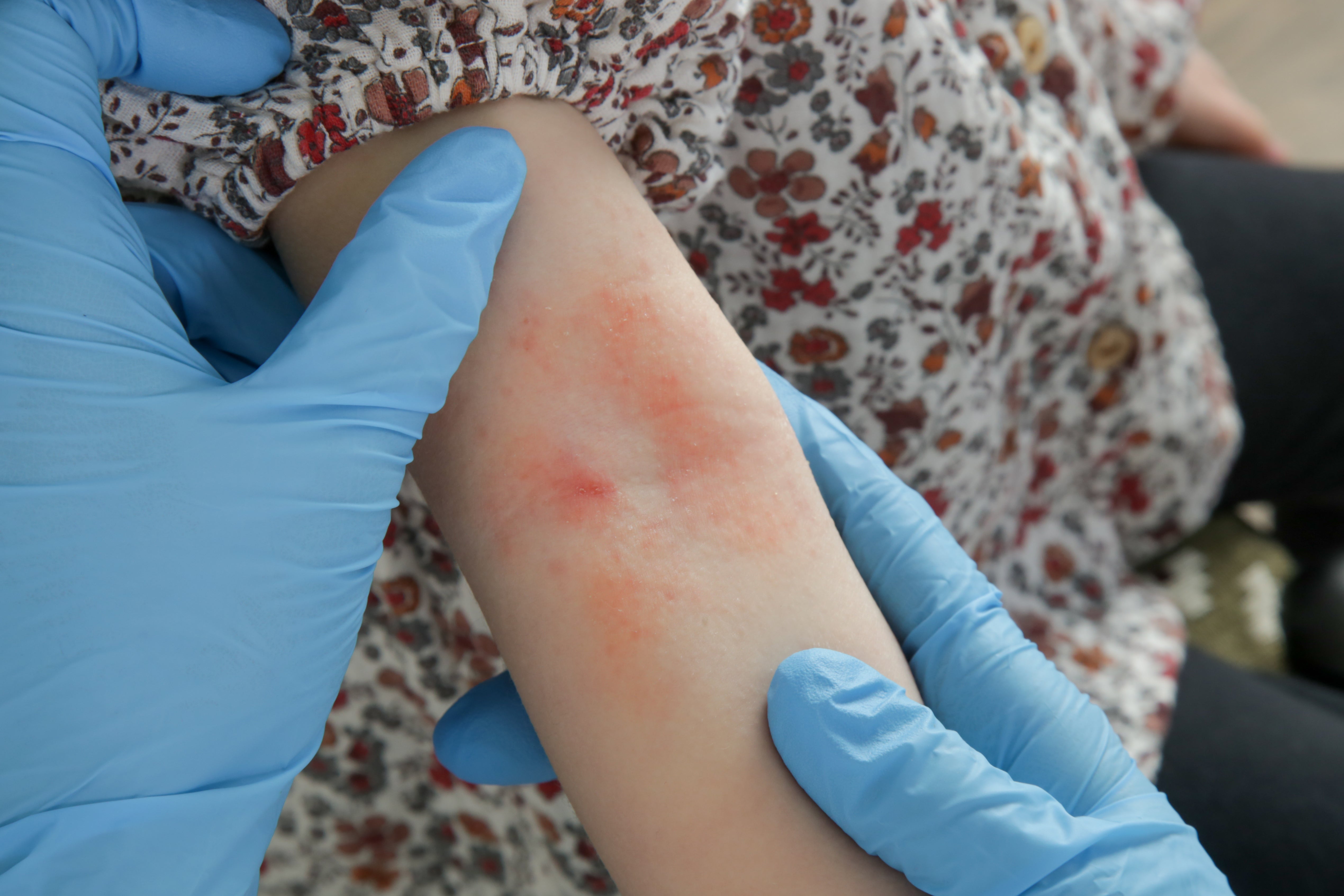
Your support makes all the difference.A new drug therapy has been shown to be “safe and highly effective” in treating moderate to severe eczema in young children.
In an international study, dupilumab was shown to reduce the severity of the skin condition within two weeks in patients aged six months to six years during clinical trials. The children and their parents also reported improved sleep and quality of life.
Based on the findings, published in journal The Lancet, doctors believe the treatment may soon be approved for children under the age of six in the UK, after it was licensed in the US in June this year.
Dupilumab is already licensed in the UK for adults and for children aged six to 18.
Dr Peter Arkwright, from the University of Manchester, who is the principal investigator for the Manchester arm of the trial, said: “Young children and infants who have moderate to severe eczema have a substantially reduced quality of life. It’s also incredibly stressful for their families, particularly as children’s sleep is so disturbed.
“The fact that infants and young children with moderate to severe eczema are inadequately controlled with creams means they have a high unmet medical need. We’re so delighted that dupilumab has provided clinically meaningful improvement, with an acceptable safety profile. These pivotal trial results strongly support the global approval of dupilumab in infants and children with eczema. It will revolutionise clinical practice worldwide.”
The phase three clinical trial, which was sponsored by biotech companies Regeneron and Sanofi, involved 162 children from around the globe, including patients at Manchester Children’s Hospital.
Of the 162 participants, 83 were given an injection of dupilumab, and 79 a placebo, every four weeks alongside the standard therapy of a low-potency steroid cream for 16 weeks.
Results showed that 28 per cent of patients receiving dupilumab achieved a global skin score of 0 or 1, indicating complete and almost complete healing of the skin at week 16.
In addition, more than half (53 per cent) of the children showed at least a 75 per cent reduction in signs of eczema and highly significant reductions in itch, alongside improved sleep.
Eczema is a condition that causes the skin to become itchy, dry and cracked. It is more common in children, often developing before their first birthday, and is usually a long-term condition, although it can improve significantly or even clear completely in some children as they get older.



Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments