Life-extending drug for incurable breast cancer approved for NHS use
Treatment could extend lives of 3,300 women each year and delay need for chemotherapy, reports Chiara Giordano
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
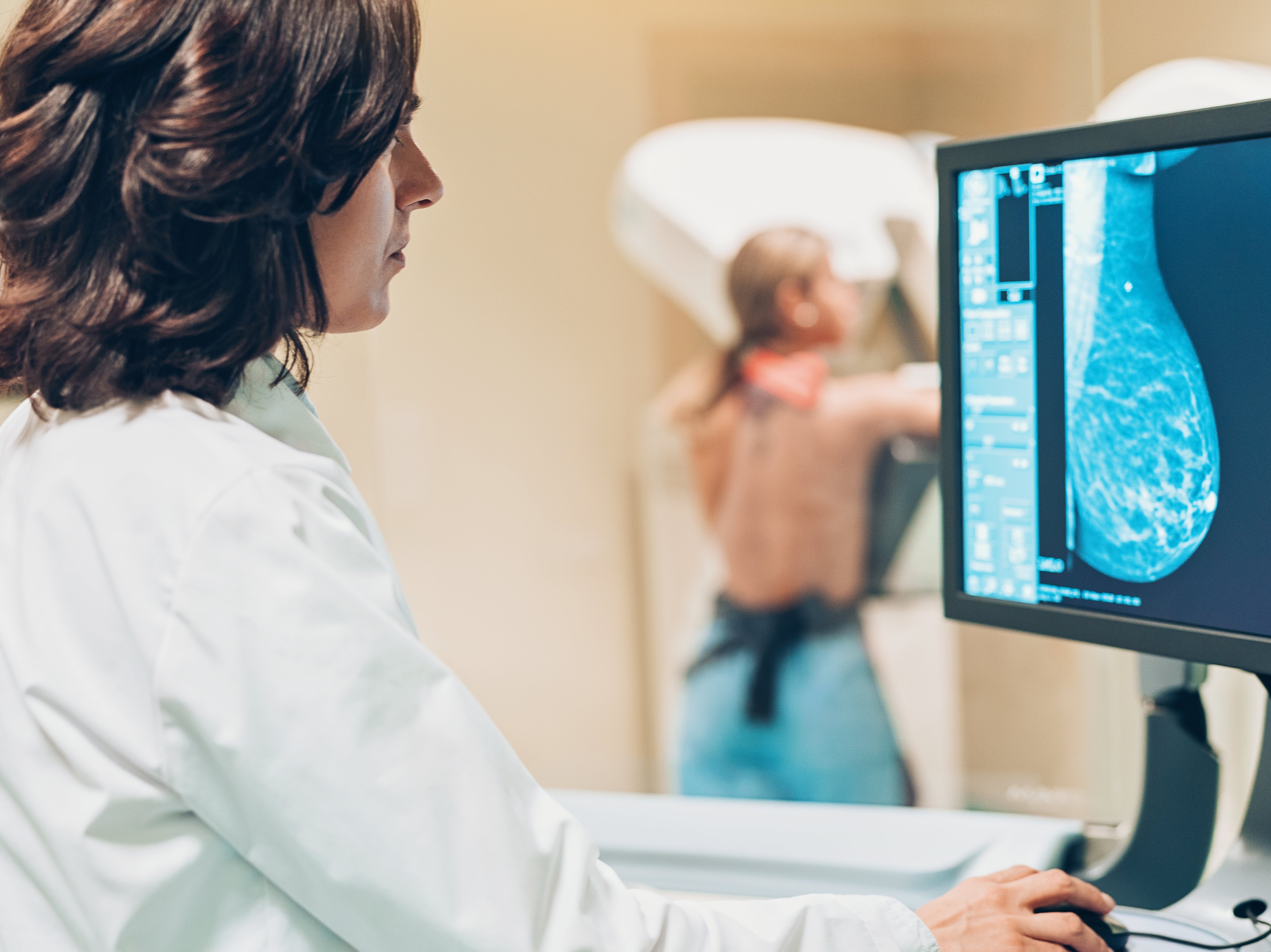
Your support makes all the difference.A drug which could help extend the lives of thousands of women with incurable advanced breast cancer has been approved for NHS use.
The National Institute for Health and Care Excellence (NICE) on Friday approved ribociclib – also known as Kisqali – for routine use by the NHS.
In its draft guidance, Nice recommended using ribociclib alongside another drug called fulvestrant to treat hormone receptor-positive, human epidermal growth factor receptor 2 (HER2)-negative, locally advanced or metastatic breast cancer.
The treatment could be an option for 3,300 women a year who have had previous endocrine therapy and where exemestane plus everolimus, another drug combination, is the most appropriate alternative treatment.
New evidence shows that, compared with fulvestrant alone, people taking ribociclib and fulvestrant together have longer before their disease gets worse and also live up to eight months longer, according to Nice.
The treatment could also allow patients to put off the point at which they start chemotherapy.
Ribociclib has been available through the Cancer Drugs Fund (CDF) since 2019, but new estimates suggest its combination with fulvestrant can be considered a cost-effective alternative to exemestane plus everolimus.
Taken once daily in pill form, ribociclib is a type of drug called a cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitor.
These work by inhibiting proteins in cancer cells, thereby preventing the cells from dividing and growing.
They are taken with an aromatase inhibitor, a type of anti-cancer drug that blocks the production of the hormone oestrogen, preventing it from stimulating the growth of hormone receptor-positive breast cancers.
A clinical trial found ribociclib and fulvestrant taken together increased median progression-free survival by 5.5 months from 9.1 months to 14.6 months compared with fulvestrant and a placebo.
Median overall survival increased by 7.7 months – from 32.5 months to 40.2 months.
Meindert Boysen, deputy chief executive and director of the NICE Centre for Health Technology Evaluation, said: “Treatments that can postpone disease progression are important because they can mean some people can avoid the often unpleasant side-effects of chemotherapy, and delay the need for its use in others.
“We are pleased therefore that our original decision to make ribociclib available through the CDF not only gave people access to it earlier than would otherwise have been possible, but has now, through the data collected during that time, allowed us to recommend it for routine use on the NHS.”
Welcoming the announcement, Baroness Delyth Morgan, chief executive of Breast Cancer Now, said: “This life-changing treatment will now bring thousands more women living with incurable secondary breast cancer hope of precious extra time to live well.
“As well as offering certain patients with incurable breast cancer extra time with loved ones, this innovative drug combination can help delay the need for chemotherapy and its debilitating side effects.
She added: “This positive news follows the recent devastating blow for patients when a similar treatment, abemaciclib with fulvestrant, was provisionally rejected.”



Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments