Why horses are bled and left to die to produce diphtheria treatment
If you were to contract diphtheria today, you would be injected with an antitoxin made with blood from horses, many of which are kept in horrific conditions in India. Steve Boggan investigates

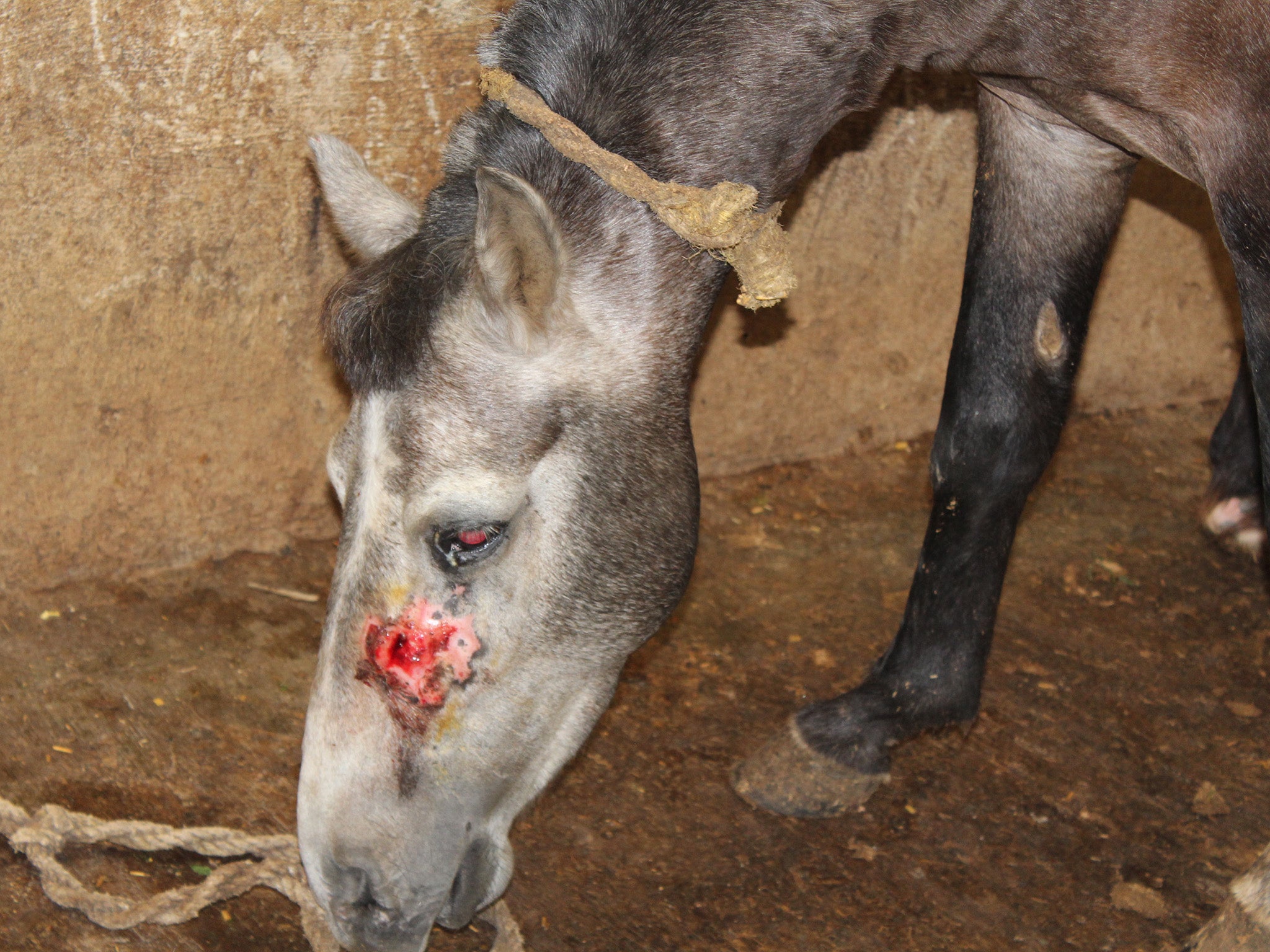
Thin and bedraggled horses, their hides caked in faeces, their skin covered in abscesses, some blind from disease, others lame and ulcerated, line up to be bled. Thick needles are inserted into their veins. Their blood pumps out, faster from the young and strong than from the old and weak, and is collected.
To the men doing the bleeding, the temptation is to take too much because this blood has value. The antibodies it contains are responsible for all but wiping out one of the greatest scourges known to humankind – diphtheria.
So revolutionary was this medical miracle – this harvesting of a serum that could cure humans of the disease – that it earned its creator, the German physiologist Emil von Behring, the first Nobel Prize for Medicine in 1901. Thanks to Von Behring, hundreds of thousands of lives a year would be saved – millions over time.
However, if you’re imagining that this equine scene is from the 1890s, when the scientist first began infecting horses with diphtheria and then collecting their antibodies, you would be wrong. Because this is how the antitoxin to fight the disease is still made.
As astonishing as it sounds, if you were to contract diphtheria today, you would be injected with an antitoxin made with blood from horses, many of which are kept in horrific conditions, mostly in India.
So successful was Von Behring, who went on to produce a vaccine for the disease, that diphtheria was almost wiped out and demand for the antitoxin – the treatment used when a person actually falls ill – fell so low that it became barely economical to make. And so, this Victorian treatment remained entrenched in the Victorian era, using ancient methodology and often poor standards of animal welfare.
But this isn’t just about the health of horses. Tens, possibly hundreds, of thousands of people contract diphtheria every year, usually in war-torn or economically and politically broken countries where healthcare systems have collapsed and vaccination programmes fallen by the wayside. Thousands of those die from the disease because supplies of the antiquated antitoxin are in short or erratic supply – and because up to 20 per cent of those treated with it suffer severe reactions to the horses’ blood plasma, a condition known as serum sickness.
A treatment made from human antibodies would have solved this problem, but because there wasn’t much money to be made from diphtheria, none was created. Now, thanks to People for the Ethical Treatment of Animals (Peta) and teams of researchers on either side of the Atlantic, two such treatments are tantalisingly close.
First, however, their inventors must overcome a seemingly insurmountable series of hurdles, including a lack of funding, the need for human trials – possibly in a war zone – and the virtual impossibility of advancing a treatment for a relatively rare disease when the world’s scientists and research laboratories are desperately focused on the search for a vaccine for Covid-19.
“We know that when we hear about 10,000 cases of diphtheria a year from the World Health Organisation (WHO), then there could be 10 times that number going unreported,” says Dr Mark Klempner of the University of Massachusetts Medical School, one of the scientists at the forefront of the quest for a new antitoxin. “The mortality rate is somewhere around 15 per cent or higher in young children, and so that’s many thousands of kids dying, and that keeps us awake at night.”
Diphtheria is an extremely contagious infection caused by the bacterium Corynebacterium diphtheriae, which is spread by coughs and sneezes. The bacterium produces a toxin that attacks cells in the membranes of the nose and throat, though in the most serious cases it also spreads to the nervous system and heart.
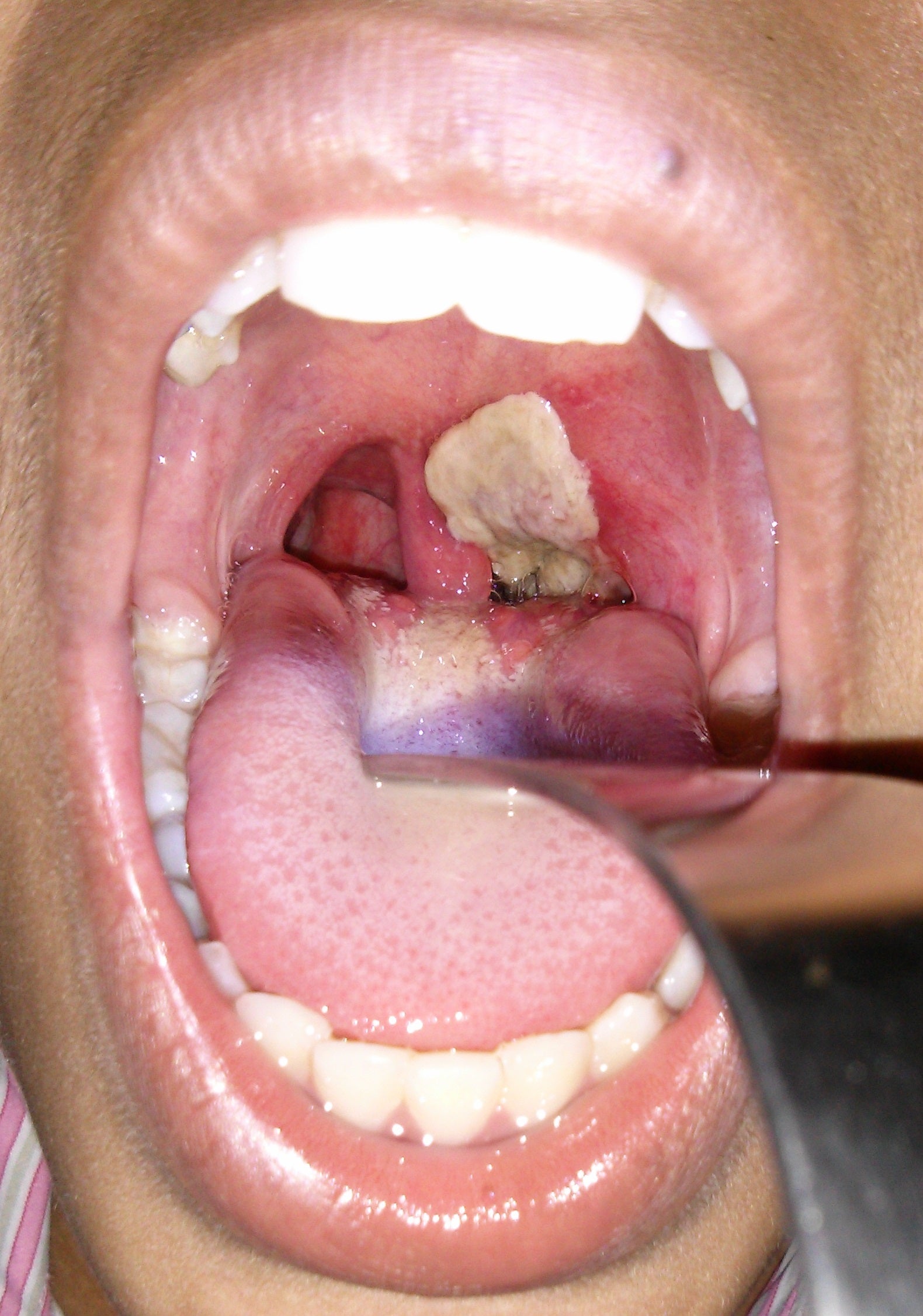
Most doctors in the UK will never have seen a case of the disease but all will know of its horrific history and the origins of its name, which is derived from the Greek word diphthera, meaning leather or hide. This is because the first visual signs of infection appear at the back of the throat as a grey, tough, build-up of dead cells that look like leather.
Victims begin by complaining of a sore throat, then they become feverish. The infection attacks the pharyngeal wall, the larynx, trachea and the bronchial tree, which distributes air to the lungs. Death is caused either by suffocation as this leather-like layer grows or by the toxin produced by the bacterium entering the bloodstream, in which case the patient may suffer paralysis and heart failure. The mortality rate for under-fives or over-40s is around 20 per cent where treatment is not available.
The oldest known reference to diphtheria-like symptoms was made by Hippocrates in his work Epidemics III in the 4th century BC. During an epidemic in the 17th century, the Spanish called the disease "the Strangler", though it later came to be known in the UK and US as “the Strangling Angel of Children”.
One facility had a chain-link cage on wheels that they rolled over horses that had fallen down rather than euthanising them [so blood could still be extracted] to keep the birds and predators from attacking them
Worldwide, hundreds of thousands of cases a year used to be reported, with tens of thousands of people, mostly young children, dying. At the turn of the last century, diphtheria was the third-highest cause of infant mortality in the UK after measles and broncho-pneumonia. Worldwide, it was second only to malaria as the most prolific killer of children. According to Oxford University’s Vaccine Knowledge Project, before a vaccination programme was introduced in the UK in 1940, diphtheria killed an average of 3,500 children a year.
During the first 10 years of the British vaccination programme, however, cases fell from 46,000 in 1940 to 962 by 1950, with the corresponding number of deaths down to just 49.
Since then, there have been very few cases in the UK and in other developed countries with functioning inoculation programmes. During the past two decades, only four people have died from the disease in the UK, and these had travelled, unvaccinated, from other countries.
However, where war or political and economic strife are prevalent, and where public health systems and vaccination programmes break down, diphtheria is never far behind. Following the break-up of the Soviet Union in the early 1990s, vaccination levels fell in its former territories, leading to a diphtheria epidemic. Between 1990 and 1998 there were 157,000 cases, leading to more than 5,000 deaths. Numbers fell only after the introduction of a massive vaccination programme across the region.
Today’s diphtheria hot spots come as no surprise: Syria, the Democratic Republic of Congo, Venezuela, Cox’s Bazar in Bangladesh, home to thousands of displaced Rohingya refugees, the Ukraine, India and Yemen.
By its nature, then, treatments for diphtheria are most needed in countries that can’t afford to pay for them, and that has made developing new treatments unattractive.
Two now on the horizon are being developed by teams at the Technical University of Braunschweig, Germany, whose work has been funded by the Peta International Science Consortium, and the University of Massachusetts Medical School, through a not-for-profit offshoot called MassBiologics. Though the American project is more advanced, having completed human safety trials of its antibody, known as S315, this is not a race, not least because neither team is seeking to make a profit from its work.
Peta began throwing funding at the problem in 2016 (it has spent €200,000 so far) in the wake of an inspection by the Animal Welfare Board of India (AWBI) of 10 Indian facilities where horse blood was being harvested. Peta had a representative on the board who recorded the appalling conditions in which the animals were kept.
Jeffrey Brown, adviser to the Peta science consortium, which, in turn, is funded by a variety of national Peta organisations, says: "We looked at 10 facilities and at those there were just under 7,000 horses and the facilities ranged from very big to very, very small. They gave a really good snapshot of conditions and there were enough of them to realise that this wasn’t a fluke – this is the state of the industry.

"Immaterial of the size of the company, the facilities looked the same, and they looked pretty bad. The horses were in a terrible shape. The most common things that you see are basic untreated injuries. Some of the regulations supposedly governing these facilities say things like ‘horses shouldn’t be too young, too old or able to get pregnant’ and you see all of those things. You see horses that are in incredibly bad condition who can no longer support their own weight, and have fallen on the ground and should be euthanised.
“There was one facility that had a chain-link cage on wheels that they rolled over horses that had fallen down rather than euthanising them [so blood could still be extracted] to keep the birds and predators from attacking them. There were a lot of abscesses, blindness, fistulas, really basic problems that horses get from standing up on hard surfaces for a long time. And these are blood collection companies, so you see a lot of signs of anaemia or other poor health indicators about body score – maybe they are too thin or bled too often, or a greater volume of blood is taken than is permitted.”
The AWBI has since been excluded from such inspections by the governmental Committee for the Purpose of Control and Supervision of Experiments on Animals, which has been criticised by animal welfare groups, fuelling suspicions that poor welfare standards continue.
Brown says the resulting medicines are often sub-standard and unreliable and, in many cases, they cause allergic reactions in humans.
“The antitoxin from horse blood is a bad product,” he says. "The medicines themselves are essential in what they are intended to be, but they are not treated with the kind of care that medical products should be. They have been left as this relic of old-fashioned approaches to medicine, and the horses are caught in the middle.
“You really don’t know what you’re getting in a little vial of this stuff. If you are talking about one single antibody, then that is called a ‘monoclonal antibody’: it is an antibody that is designed to bind to one very specific molecular link. If you are using monoclonal antibodies in the laboratory, they are sold as, ‘this antibody binds to, or sticks to X’, whatever X is, and the assumption for quite a long time has been ‘Well, we can trust that labelling’. But research [at the Technical University of Braunschweig] has established that fully one-third of these animal-derived supposed monoclonal antibodies stick to things that they are not advertised as sticking to.
"So, when we are talking about antitoxins that are derived from horse blood, those aren’t monoclonal antibodies. That is absolutely a mixture, a completely unknown uncharacterised mixture, of thousands or millions of different antibodies. If among them are some antibodies that can neutralise the diphtheria toxin, then the product can be approved.
“It’s scattershot in more ways than you can imagine. These products aren’t standardised; they are made from many living animals and they are produced in batches, so from batch to batch to batch over time the animals are different, the health conditions of the animals are different, so the quality, contents of the product changes over time. Even though a company might say ‘We make diphtheria antitoxin – you can buy it from us all the time’, every single vial of that equine antitoxin is different every time.”
One of the 10 producers criticised after the 2016 inspections was VINS BioProducts Ltd of Hyderabad, where, according to Peta, experts found “equines with eye abnormalities, equines with open wounds and lesions, improper animal husbandry techniques, and lack of euthanasia for suffering and dying animals”.
The company claims welfare is now a priority.
“Our animals are stabled and fed according to strict welfare regulations,” said Ajit Nair, a director of the company. “We are subject to regular inspections by the Committee for the Purpose of Control and Supervision of Experiments on Animals and any violations of their regulations would be punished.”
In a 2017 report entitled Diphtheria antitoxin supply issues, the WHO flagged up its scarcity and identified only a handful of small companies worldwide that made insufficient quantities on an irregular basis.
“A few countries have been maintaining stockpiles,” the report reads. "However, these stockpiles are small and most have either expired or have had the expiry dates extended through re-testing by an independent laboratory to confirm activity of the product.
“Effective treatment of cases requires rapid access to and administration of diphtheria antitoxin. In the event of a suspected or confirmed case in the absence of a stockpile the time taken to identify a manufacturer with remaining supply within expiry date (if at all available), and the time to arrange for shipment, may mean the supply arrives too late to save the lives of the patients.”
Dr Klempner of MassBiologics says his laboratory was originally established 125 years ago to make diphtheria antitoxin from equine antibodies, and now it has gone full circle. Earlier this year, the not-for-profit company successfully completed safety trials of a human monoclonal antibody harvested from human volunteers who had been vaccinated against diphtheria.
Where one treatment exists – in this case, the horse antitoxin – it is difficult to find people prepared to fund development of another
“This is an effective treatment – we know it is,” he says. “We have now completed the first-in-human trials where we have given it to volunteers, shown that it is perfectly safe and achieves neutralising levels against the diphtheria toxins that far exceed the neutralisation of the toxin by horse serum. So, here is a safer, more potent medicine that we are desperate to get out there.”
The problem is that “out there” is likely to be a war zone or a failed state where conditions for a trial could be difficult at best, dangerous at worst. The company has held talks with WHO, the Pan American Health Organisation and Médecins Sans Frontières (MSF) about helping with frontline trials, but so far no further trials are in place.
Julien Potet, MSF’s policy adviser on vaccines, says: “As you can imagine, testing new drugs in humanitarian settings presents some specific challenges. We are discussing MassBiologic’s proposal internally among our different operational centres.”
The University of Braunschweig’s methodology under Professor Michael Hust and Dr Esther Wenzel was different from MassBiologics, yet their hurdles remain similar.
They used a gene technology approach called “phage display”, which involved three donors being given a diphtheria immunisation. Blood taken from them was then used to isolate the immune cells that produce antibodies that attack the diphtheria toxin. The genes encoding these antibodies were cloned into “phage vectors” [phages are viruses that infect bacteria] to build a library of millions of phages, with each phage having a different antibody on its surface. Those that bound most tightly to the diphtheria toxin were then selected, produced and analysed. Finally, two or three different antibodies were combined to make the most effective antitoxin.
The Braunschweig team was ready to move on to the next step, trials in humans, when Covid-19 struck and, in common with labs all around the world, their work was re-directed to focus on that.
“We wanted to talk to the Bill and Melinda Gates Foundation, the European & Developing Countries Clinical Trials Partnership, the National Institutes of Health [in the US], and the Wellcome Trust [in the UK],” says Professor Hust. “But then we all got involved in coronavirus research so it hasn’t happened.”
Even when the researchers are able to turn their attention back to the diphtheria antitoxin, they will have their work cut out; the trials needed to take a treatment to approval level traditionally cost millions of dollars. And where one treatment exists – in this case, the horse antitoxin – it is difficult to find people prepared to fund development of another.
“It is difficult to convince people and institutions to go on with further development because the argument is that the horse sera are working, they are available and when you would like to bring a new drug on to the market, you have to show efficacy – and you have to compare it with the horse sera,” says Hust.
“Of course, the horse sera are not approved drugs because they have been used for more than a hundred years. They were not approved drugs, yet you have to compare any new drugs with them.”
For now, perhaps for the foreseeable future, that is where this story ends. Nobody would argue that the search for a Covid-19 vaccine must take priority, yet the fact that children are dying because of a shortage of an antiquated antitoxin when a potentially new and safer one is almost at hand is desperately saddening.
As Dr Klempner says, it is enough to keep you awake at night.




Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments