The key to staying forever young could reside in a lab in Cambridge
Andy Martin would like to be 39 again. He talks to Wolf Reik about epigenetics, the science of cellular rejuvenation, and getting old without getting older


Imagine a terminator coming back through time to get you. It is programmed to cut you off in your prime, to stop you in your tracks before you can really get going. You are, in a word, doomed. Well, the good news is that you don’t have to imagine this apocalyptic scenario – because it really is happening, right now. And the sad truth is that the terminator is not just real, the terminator is you. Whether you like it or not, you will end up terminating yourself. And there’s not a thing you can do to stop this monstrous killing machine carrying out its mission. Or is there?
Wolf Reik leads me to think that it might be possible, in the final reel, to fight off the Grim Reaper that is within you. I’ve always liked the story of King Canute who planted his throne on the beach right where the waves are breaking and had a crack at holding back the tide. And of course failed (in the smart version of the legend he is proving to his incredulous followers that he is not in fact omnipotent and even he must submit to the greater power of nature). Something similar must apply, surely, to the tide of time: none of us can hold that back either. But maybe Reik has done just that.
Reik is the director of the Babraham Institute, the world-class centre of research into the mechanisms of ageing and rejuvenation, located in the hills around Cambridge, and a professor of epigenetics (which is, in a nutshell, everything that impacts on the genome that is not strictly genomic, the realm where nurture meets nature). And he is a keen cyclist too. He stresses that, for the time being, probably the best thing you can do to “slow down” the advancing tide is (a) cycle more (b) get a good night’s sleep (c) eat your greens and (d) brush your teeth properly (it’s true – good oral hygiene is crucial).

But, assuming you have already taken all those essentials on board, what you need to know is that Reik (along with a labful of fellow Babraham researchers, including notably PhD student Diljeet Gill) has recently been working on what he calls “transient cellular reprogramming”. Which may just be – if you would prefer not to terminate yourself prematurely – the answer to your prayers. In fact he’s done more than hold back the tide: he has rewound the clock of the human body. “It’s all reversible,” he says. “In principle.”
He has succeeded in knocking 30 years off the age of some creaky, wrinkly old cells. One day, perhaps, you (having attained a certain age) will be able to walk into Reik’s clinic and he’ll say: “What age would you like to be?” And you’ll say: “How about 39?” And he’ll say: “Let’s make it 38, shall we, just to be on the safe side.” It’s the theory of sustainability, but applied to human beings. Reik, by the way, is 63, chronologically speaking, but he looks as if he’s rewound a decade or so. For all I know he could be like the High Lama of Shangri-La and live to 200 and choose to stop his pulse whenever he’s had enough.
My basic assumption is that everything always goes wrong. This turns out to be scientifically correct. Especially where the human body is concerned. We have built-in obsolescence. The science of exactly how it is we go steadily downhill has advanced in leaps and bounds over the last few decades (and has been brilliantly documented in Andrew Steele’s recent book, Ageless). The right way to think about ageing is not in terms of your hairline receding or the wrinkles on your forehead deepening or your running just a little more slowly this year than you did last year. That is not science, that is just you. The science of human entropy begins at the micro- or nano-level with the cell.
The Who (now getting on a bit) once sang ‘I want to die before I get old’. Reik is a bit like that: he is not offering immortality but rather rejuvenation, the science of ‘getting old without getting older’
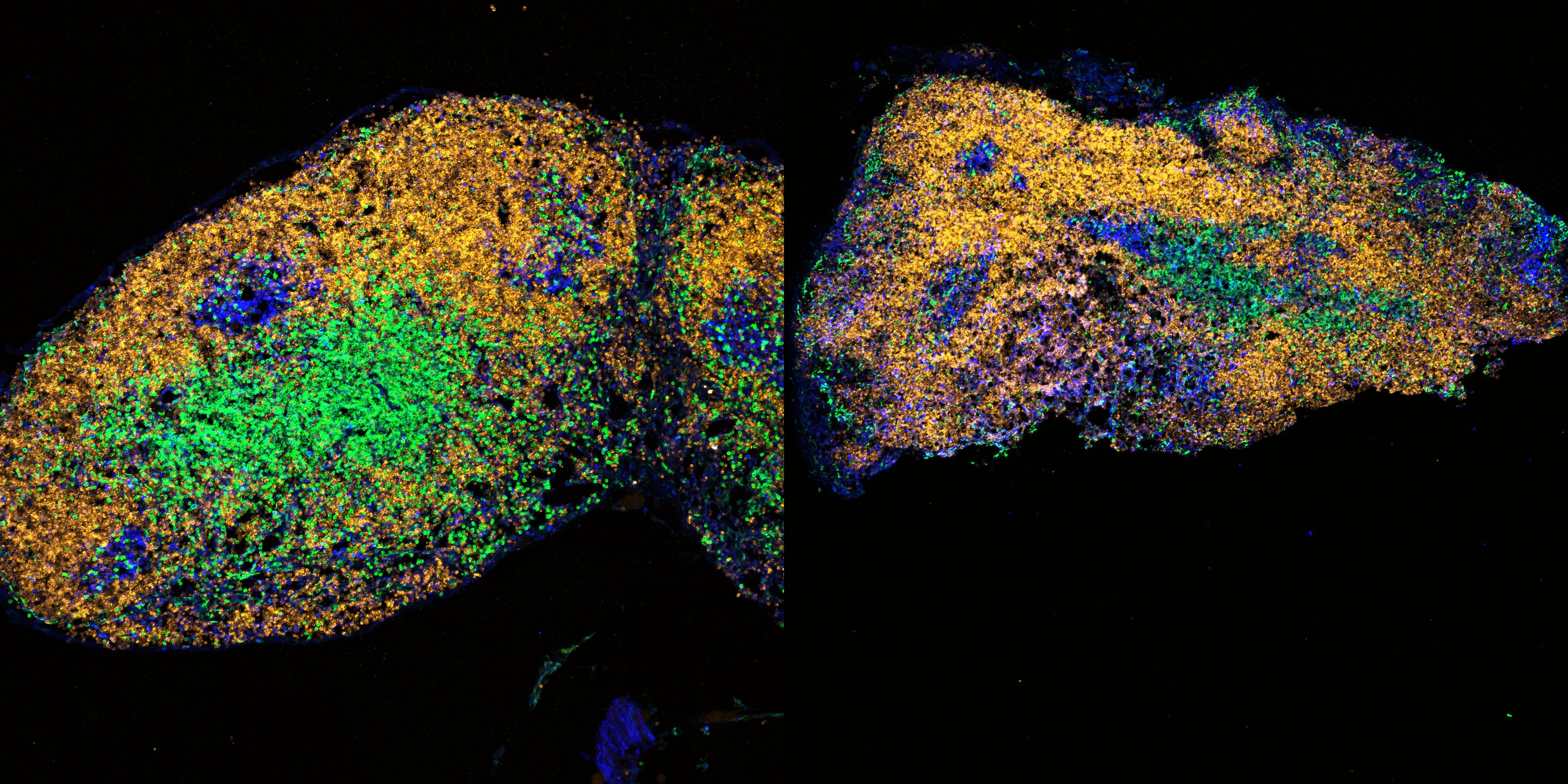
The Babraham Institute, part of the Babraham Research Campus, is the Cern of human biology. They do what they call “fundamental science” (which means it’s going to take a little while before you can walk into Boots and pick up your prescription). Whereas at CERN in Geneva you can follow the tracks left by extremely speedy and otherwise elusive particles (such as the Higgs boson or “God particle”), at the Institute’s Imaging Facility you can look down the barrel of an electron microscope and survey the wondrous enigma of the cell.
The human body consists of trillions of them. You’re looking at the primal constituents of life. I was reminded, peeking into Dr Simon Walker’s electron microscope, of gazing through a telescope on top of a mountain in Hawaii at the most remote star we can see in the universe: it’s like looking back through time at the Big Bang. At this resolution, the cells of all organisms look curiously alike: zoom in tight enough and a human looks like a mouse which looks like a nematode worm. “It shows,” says Walker, “how we’re all related – it’s like you’re looking back at the common ancestor, before we became multi-cellular organisms.”
And, if you look very closely, you can see why this very nice young cell, bursting with life and youthful enthusiasm, is going to die. You can, in effect, read the palm of a cell. Your destiny is written in your epigenetic information. “It’s not wear and tear,” says Reik. “It’s programmed.” One student of Reik’s went off and set up a separate spin-off company that can take a peak at your epigenetic clock and give you a confident life-expectation prediction (they’re called Chronomics if you want to find out how long you’ve got left – and what you can do about it).
In trying to fix everything that goes wrong at one end of life, Reik has been going back to what goes right at the other end, at the very beginning of the formation of life. You, the embryonic you, originated as a single cell or “zygote”. The Romeo and Juliet of sperm and egg have had their moment of fun. And out of that close encounter emerges one entirely new life form, never before seen in the history of the universe (while nevertheless bearing a strong resemblance to its precursors). Way back when, several billions of years ago, a single cell not wholly unlike this one, was born out of the great chemical soup that was the earth. And then it divided and became two cells. Division is a form of multiplication. So too you, at the very beginning of your journey, start dividing and multiplying: into two, then four, then eight and so on, cloning and re-cloning yourself, until you are born, and then you keep on going, dividing and multiplying and cloning until the day you die.
It’s a system that works well enough for a while, and then it breaks down. Because, as Reik puts it: “It’s programmed to make mistakes at every cell division.” It seems like we are designed to fail. The photocopier is always going to get jammed or run out of ink in the end. Fortunately, Reik is like your friendly Xerox engineer who can fix the biological photocopier that is you.

This is how it all goes wrong: at the tail ends of your chromosomes you have these little caps known as “telomeres”. Every time your cells divide we make copy-and-paste mistakes and the telomeres get slightly trimmed or shortened. If you keep on copying long enough you’re going to run out of telomeres – “the information content degrades” as Reik puts it, “and your cells begin to function sub-optimally”. They become “senescent”. Mostly the bad old cells commit suicide or they are offed by passing assassin cells. “Apoptosis”, the death of cells, is a good thing. Ironically, the trouble starts when they don’t die properly but turn into rogue or zombie cells. Eventually they accumulate and your system sinks under a pile of junk. Finally, it’s curtains.
There are things you can do to prolong organic life. For example, in the last century it was discovered that a “restricted diet” enabled rats to live longer. This is fact: the rats who are allowed to stuff themselves conk out a whole lot sooner than the rats who are prevented from constant gorging. It’s good to know, but no one really wants to half-starve themselves if they don’t have to.
At the most fundamental level, that dodgy old cell that is on the verge of malfunction can – says Reik – be restored to what it once was. The artist formerly known as a stem cell. The epigenetic clock is set back to zero. The great thing about the stem cell is that it can do anything, become anything: this stem cell goes off to become a heart cell, that one goes off to become part of the brain, and so on. As they get older they split off and “differentiate”, taking up specific jobs – and eventually get worn out from doing the job. But with the injection of a small amount of the elixir known as “Yamanaka factors” (a gene cocktail) they can be turned back into the all-singing all-dancing do-anything Renaissance-man type cells that they once were (aka “induced pluripotent stem cells”).
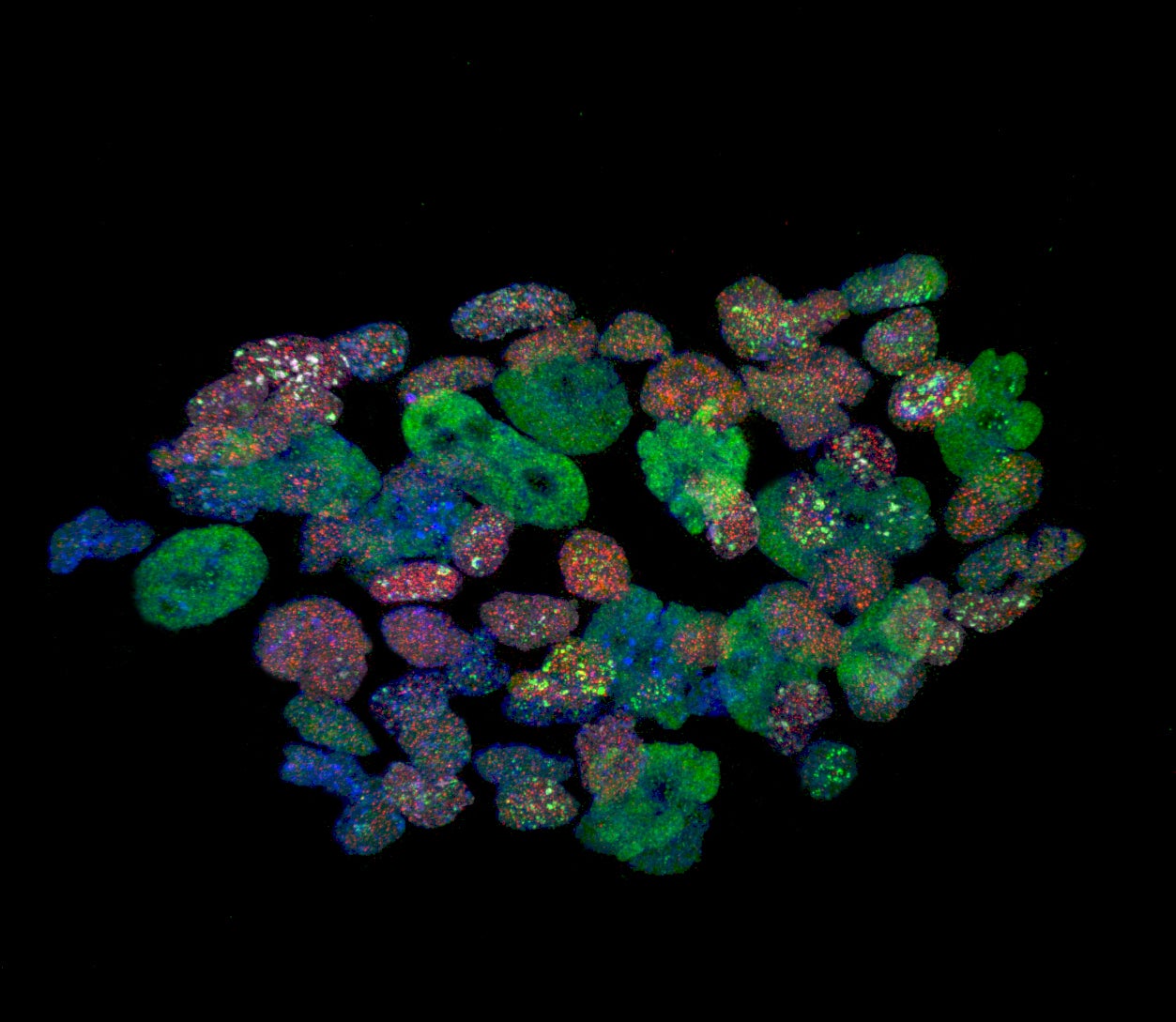
Which sounds fine until you stop to consider: you can take out all your old cells, renew and regenerate, make them born-again, but then… who’s getting your heart to pump, what about all the old brain cells, not to mention knees, eyes, mouth etc? Those hard-working cells can’t all go off on holiday and just sit there, quietly rejuvenating. Not unless you want to go back to being an embryo, made up of undifferentiated cells, like a tabula rasa. Or find out later that your good old skin cell has decided to turn into a follicle or a finger nail.
Now Reik and his team have worked out a way to reprogramme the cells, to renew and recharge, but also get them back on the job, thus pulling off a massive double trick, taking, say, an old skin cell and flipping it into a stem cell (“switching it on”), but then re-flipping it back into a skin cell once more (“switching it off”), but a brand new one, better than ever. Then all you need to do is re-insert that cell where it came from and let it re-populate and thereby regenerate.
And what about a bit of brain maintenance while we’re at it? Shove it in for an MOT before the lights go out. In the more immediate future it is possible to envisage growing new skin in the treatment of burns
Let’s say, for example, you have a dodgy heart. What are you going to do? You can take out the old one and replace it with a new one: it’s tricky and a little barbaric, but it can be done. It’s basic engineering. But what about if you take out, instead, a few heart cells, restore them to their optimal state, and stick them back in again? Wouldn’t that be a whole lot easier? Similarly with all those old knee and hip replacement operations. And what about a bit of brain maintenance while we’re at it? Shove it in for an MOT before the lights go out. In the more immediate future it is possible to envisage growing new skin in the treatment of burns. In the future surgery will be all about cellular engineering.
Part of the job at the Babraham is toughening up the immune system and working out why it tends to decline in resilience with age. This is the realm of the immunology department and Dr Michelle Linterman. She knows that your immune cells find it harder to fight off those unwanted invaders as you get older – and you end up with chronic inflammation or “inflammaging”). “There is no evolutionary pressure to achieve longevity,” she points out. In other words, once you have performed your mission of passing on your genes (or not), your time is up. Tragically, despite all the awe-inspiring work at the Babraham and other research labs around the world, it wasn’t enough to save the life of 65 year-old Michael Wakelam, the Institute’s previous director, when he contracted Covid in March of 2020. “We didn’t know then what we know now,” says Linterman.

The Babraham Institute is all about increasing knowledge. “He that increaseth knowledge increaseth sorrow” says the Ecclesiast, very wisely. But it’s possible that increasing knowledge could also, ultimately, reduce sorrow. Reik and the Babraham are not talking about increasing life span. They don’t buy into the theory of the 1,000-year old man, beloved of Aubrey de Grey (founder of Sens research, “Strategies for Engineered Negligible Senescence”) and certain Silicon Valley bio-hackers. Their priority is what they call “health span”: they want you to be in good nick until the day you finally flake out. The Who (now getting on a bit) once sang, “I want to die before I get old.” Reik is a bit like that: he is not offering immortality but rather rejuvenation, the science, in Andrew Steele’s phrase, of “getting old without getting older” – the possibility, as James Dean put it so neatly, of being “a good-looking corpse”.
Take the case of an old friend of mine, now in her nineties. Still as sharp as ever, but increasingly immobile, verging on being unable to do much at all under her own steam any more. She is cared for, but she hates not being autonomous. With the application of “Wolf-therapy”, she could one day be back out there walking the dog and doing the shopping for herself. As one of the Babraham crew predicted, “nonagenarians will go solo sailing”.
As I was cycling back from Babraham (following Reik’s wise advice), I got a text from Jonathan, an old friend. Yes, emphasis on the old. He said: “I need some of that. Whatever it is.” There he speaks for an entire (older) generation. And, as The Who would say, I’m talkin’ ‘bout my generation. I wouldn’t say no to a bit of transient cellular re-programming myself. If they want a guinea pig or, you might say, a Wolfman, I’m in.
Andy Martin is the author of ‘Surf, Sweat and Tears: The Epic Life and Mysterious Death of Edward George William Omar Deerhurst’ (OR Books)
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments