National HIV Testing Week: How self-testing kits will help to end the epidemic by 2030
Brigette Bard, founder of BioSure, tells Zlata Rodionova that the kit helps to normalise the conversation around the virus so more people can be protected

The next time you stop at your local Boots store to buy household staples like soap, shampoo or tissues, you might also consider picking up something more unconventional but nonetheless essential: an HIV test
“HIV is treatable and no longer a death sentence for those who know they have it. The fact that you can now find self-testing kits on shelves helps to normalise the conversations around it so more people can be protected,” says Brigette Bard, the founder of BioSure, the start-up which began manufacturing the first legally approved HIV self-testing kit in the UK in 2015, following the overturn of the legislation banning such devices.
In England, while new HIV diagnoses have fallen to their lowest in almost two decades, the latest data from Public Health England reports that 43 per cent of all diagnoses were late.
A diagnosis in the later stages of the infection not only means that people are less likely to recover from the virus, but also that they have more opportunity to unknowingly infect others. Early detection could prove life-saving for many.
With ever-mounting pressure on the NHS, the theory is home self-testing kits should relieve pressure on overcrowded sexual health clinics but also encourage more people to get tested, increase early diagnosis rates and contribute in ending the epidemic by 2030.
Bard, who sold her first brokerage company to launch BioSure, says: “As a woman, I thought there would be no way that I would want to get a doctor’s appointment, take time off work and have some questions asked of me – self-testing has always been a complete no-brainer.”
“In the early 1990s, HIV was considered a death sentence, but now antiretroviral treatments are so good it can bring the virus to ‘undetectable levels’. It means people can lead a normal life and crucially they can’t pass on the virus, so we needed a dynamic change in how people could get tested and diagnosed.”
Creating the kit was a long process that took about two years. The stigma around the virus meant the UK was only the second country in the world to change its legislation to permit the marketing and sale of HIV self-tests. Meanwhile, retailers were worried about their reputation and negative PR
Bard explains: “Policymakers and retailers were both terrified of it. We had to do a lot of education around it. The concerns were around people not referring into care after using the test, whether the accuracy of the tests could achieve the same levels of the ones undertaken by a professional and making sure there was no evidence of it linking to any self-harm.

“In a way, a lot of these concerns were also raised with pregnancy tests and women finding out they were pregnant on their own – but really it’s an empowered choice and it’s about taking control of your own health.”
Four years later, although unfounded prejudices around HIV still remain, attitudes are slowly beginning to change.
More than 800 Boots branches are now stocking HIV self-test kits, which cost £33.95.
Superdrug was the first retailer to make the tests available across its 200 pharmacies in 2018, while Lloyds Pharmacy now sells them via its website.
According to Bard, about 100,000 kits were sold in the UK so far this year.
Demand for the DIY tests in South Africa has also grown month-on-month since 2015, and combined with other markets such as Brazil, Ethiopia, Kenya and Hong Kong among others, exporting accounts for more than 40 per cent of the firm’s sales. This figure is only set to grow as the business is looking to expand into new markets in Asia next year.
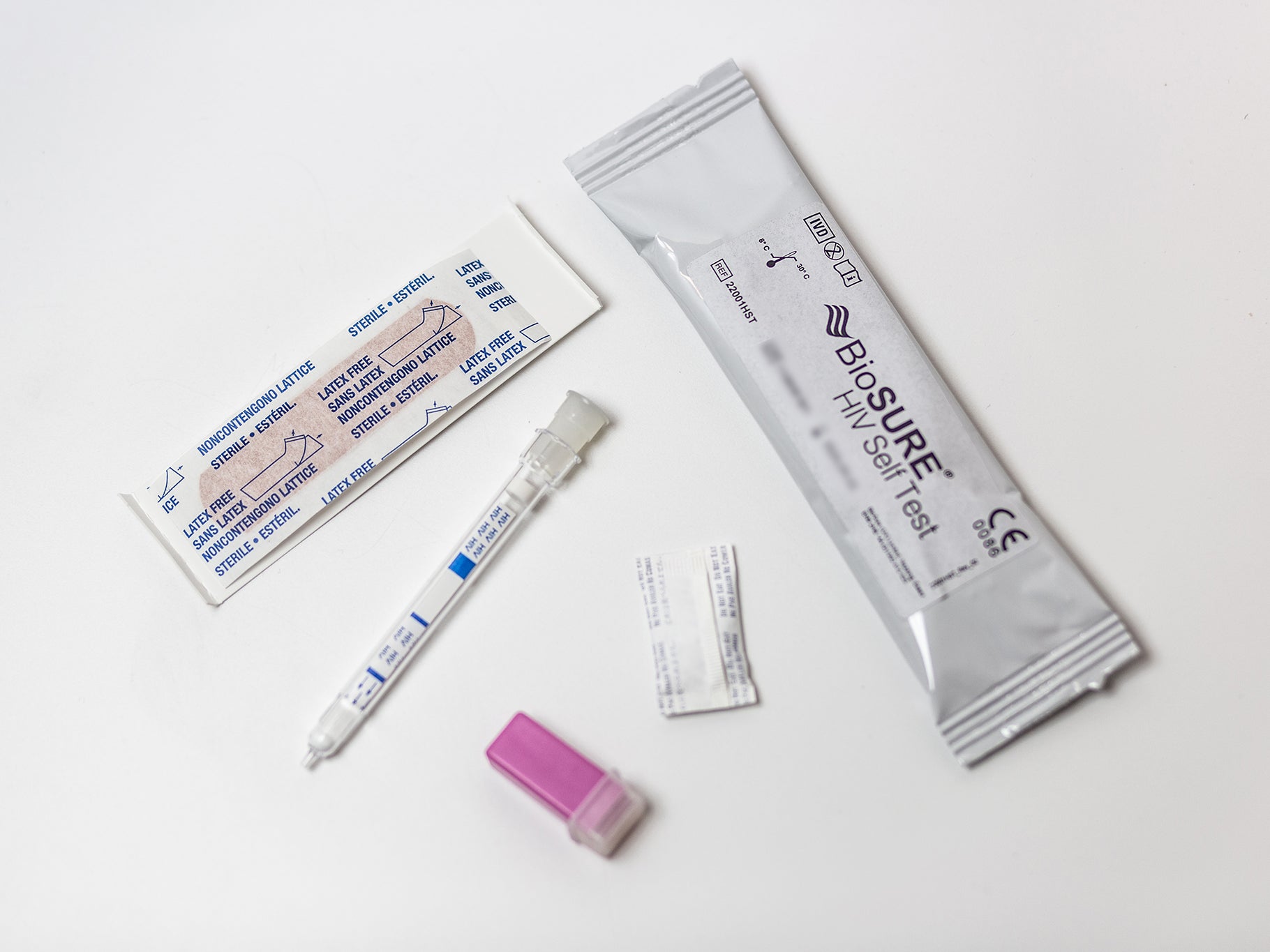
So how does a BioSure test actually work? All you need to do is prick your finger, add the sample to the testing device, wait 15 minutes and get your “initial result”.
In a way, a lot of these concerns were also raised with pregnancy tests and women finding out they were pregnant on their own – but really it’s an empowered choice and it’s about taking control of your own health
Despite the device’s 99.7 per cent accuracy, any positive result would need to be confirmed in a lab, Bard insists.
Crucially, a negative line can only be triggered if there is enough blood on the test, meaning people know immediately if they’ve done the testing correctly so they can trust their result.
In the US, a similar test has been available since 2012 – but Bard argues the is taken from a sample of the person’s saliva making it less accurate.
“We are still small, and up against multibillion-dollar companies, but I’d like to think that we generate lots of positive conversations and hopefully educating people that way.
“Increasing the testing and making it more accessible means more people get treatment and more people don’t transmit – that’s really our biggest driver, the fact that we are part of the solution in ending this epidemic by 2030.”
In 2018, new infections among young women aged 15 to 24 were 55 per cent higher than among men of the same age group.
Bard explains that part of the reason is that women are routinely screened for HIV in the UK when they access maternity services while men don’t necessarily engage with healthcare.
Spreading the word about testing across the board for anyone who has taken a risk as opposed to some specific population is the only solution to curb these numbers, according to Bard.
“There are still a lot of stereotypes surrounding HIV and people are sometimes lulled into a sense of security when they are still at risk. It’s now easier to get access to a test, use it and get support if you get a positive result. I want people to take control over their own health.”
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments